Intestinal Bacteria Encapsulated by Biomaterials Enhance Immunotherapy
- PMID: 33643302
- PMCID: PMC7902919
- DOI: 10.3389/fimmu.2020.620170
Intestinal Bacteria Encapsulated by Biomaterials Enhance Immunotherapy
Abstract
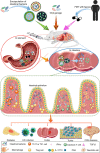
The human intestine contains thousands of bacterial species essential for optimal health. Aside from their pathogenic effects, these bacteria have been associated with the efficacy of various treatments of diseases. Due to their impact on many human diseases, intestinal bacteria are receiving increasing research attention, and recent studies on intestinal bacteria and their effects on treatments has yielded valuable results. Particularly, intestinal bacteria can affect responses to numerous forms of immunotherapy, especially cancer therapy. With the development of precision medicine, understanding the factors that influence intestinal bacteria and how they can be regulated to enhance immunotherapy effects will improve the application prospects of intestinal bacteria therapy. Further, biomaterials employed for the convenient and efficient delivery of intestinal bacteria to the body have also become a research hotspot. In this review, we discuss the recent findings on the regulatory role of intestinal bacteria in immunotherapy, focusing on immune cells they regulate. We also summarize biomaterials used for their delivery.
Keywords: biomaterial; immune cell; immunotherapy; intestinal bacteria; oral delivery; probiotic.
Copyright © 2021 Liu, Li, Wu, Jing, Li and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer XW declared a shared affiliation with several of the authors YL, ZL, YW, LL, XJ, XF, to the handling editor at time of review.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

