A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution
- PMID: 33643307
- PMCID: PMC7902526
- DOI: 10.3389/fimmu.2020.631319
A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution
Abstract
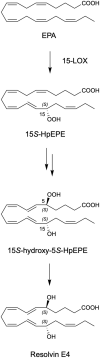
The resolution of the acute inflammatory response is governed by phagocytes actively clearing apoptotic cells and pathogens. Biosynthesis of the specialized pro-resolving mediators (SPMs) is pivotal in the resolution of inflammation via their roles in innate immune cells. Resolvin E4 (RvE4: 5S,15S-dihydroxy-eicosapentaenoic acid) is a newly uncovered member of the E-series resolvins biosynthesized from eicosapentaenoic acid (EPA) recently elucidated in physiologic hypoxia. This new resolvin was termed RvE4 given its ability to increase efferocytosis of apoptotic cells by macrophages. Herein, we report on the total organic synthesis of RvE4 confirming its unique structure, complete stereochemistry assignment and function. This synthetic RvE4 matched the physical properties of biogenic RvE4 material, i.e. ultra-violet (UV) absorbance, chromatographic behavior, and tandem mass spectrometry (MS2) fragmentation, as well as bioactivity. We confirmed RvE4 potent responses with human M2 macrophage efferocytosis of human apoptotic neutrophils and senescent red blood cells. Together, these results provide direct evidence for the assignment of the complete stereochemistry of RvE4 as 5S,15S-dihydroxy-6E,8Z,11Z,13E,17Z-eicosapentaenoic acid and its bioactions in human phagocyte response.
Keywords: M2 macrophages; eicosapentaenoic acid; lipid mediator; omega-3 fatty acids; phagocytes; pro-resolving; pro-resolving mediator.
Copyright © 2021 Libreros, Shay, Nshimiyimana, Fichtner, Martin, Wourms and Serhan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

