Role of Human Milk Bioactives on Infants' Gut and Immune Health
- PMID: 33643310
- PMCID: PMC7909314
- DOI: 10.3389/fimmu.2021.604080
Role of Human Milk Bioactives on Infants' Gut and Immune Health
Abstract
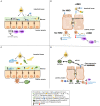
Exclusive human milk feeding of the newborn is recommended during the first 6 months of life to promote optimal health outcomes during early life and beyond. Human milk contains a variety of bioactive factors such as hormones, cytokines, leukocytes, immunoglobulins, lactoferrin, lysozyme, stem cells, human milk oligosaccharides (HMOs), microbiota, and microRNAs. Recent findings highlighted the potential importance of adding HMOs into infant formula for their roles in enhancing host defense mechanisms in neonates. Therefore, understanding the roles of human milk bioactive factors on immune function is critical to build the scientific evidence base around breastfeeding recommendations, and to enhance positive health outcomes in formula fed infants through modifications to formulas. However, there are still knowledge gaps concerning the roles of different milk components, the interactions between the different components, and the mechanisms behind health outcomes are poorly understood. This review aims to show the current knowledge about HMOs, milk microbiota, immunoglobulins, lactoferrin, and milk microRNAs (miRNAs) and how these could have similar mechanisms of regulating gut and microbiota function. It will also highlight the knowledge gaps for future research.
Keywords: breastmilk; development; gut; human milk; immune system; immunity; infants; neonates.
Copyright © 2021 Carr, Virmani, Rosa, Munblit, Matazel, Elolimy and Yeruva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

