Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases?
- PMID: 33643313
- PMCID: PMC7907174
- DOI: 10.3389/fimmu.2021.625993
Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases?
Abstract
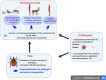
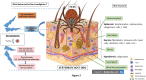
Ticks and tick transmitted infectious agents are increasing global public health threats due to increasing abundance, expanding geographic ranges of vectors and pathogens, and emerging tick-borne infectious agents. Greater understanding of tick, host, and pathogen interactions will contribute to development of novel tick control and disease prevention strategies. Tick-borne pathogens adapt in multiple ways to very different tick and vertebrate host environments and defenses. Ticks effectively pharmacomodulate by its saliva host innate and adaptive immune defenses. In this review, we examine the idea that successful synergy between tick and tick-borne pathogen results in host immune tolerance that facilitates successful tick infection and feeding, creates a favorable site for pathogen introduction, modulates cutaneous and systemic immune defenses to establish infection, and contributes to successful long-term infection. Tick, host, and pathogen elements examined here include interaction of tick innate immunity and microbiome with tick-borne pathogens; tick modulation of host cutaneous defenses prior to pathogen transmission; how tick and pathogen target vertebrate host defenses that lead to different modes of interaction and host infection status (reservoir, incompetent, resistant, clinically ill); tick saliva bioactive molecules as important factors in determining those pathogens for which the tick is a competent vector; and, the need for translational studies to advance this field of study. Gaps in our understanding of these relationships are identified, that if successfully addressed, can advance the development of strategies to successfully disrupt both tick feeding and pathogen transmission.
Keywords: adaptive immunity; immune tolerance; innate immunity; skin immunity and microbiome; tick; tick-borne diseases.
Copyright © 2021 Boulanger and Wikel.
Conflict of interest statement
SW is Senior Science Advisor for U.S. Biologic, a biotechnology company that develops solutions for tick-borne diseases, pet health, and antimicrobial resistance in poultry and livestock through oral vaccines. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

