Bone Fragment Co-transplantation Alongside Bone Marrow Aspirate Infusion Protects Kidney Transplant Recipients
- PMID: 33643315
- PMCID: PMC7904687
- DOI: 10.3389/fimmu.2021.630710
Bone Fragment Co-transplantation Alongside Bone Marrow Aspirate Infusion Protects Kidney Transplant Recipients
Abstract
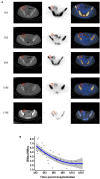
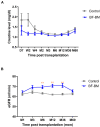
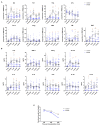
Integration of non-vascularized bone grafting and bone marrow aspirate infusion in transplantation may provide clinical benefit. Here we have incorporated bone fragment co-transplantation and bone marrow aspirate infusion (BF-BM) into living kidney transplantation (LKT). Twenty LKT recipients receiving bone fragments and bone marrow aspirates donated from their corresponding donors were enrolled into a retrospective study. A contemporaneous control group was formed of 38 out of 128 conventional LKT recipients, selected using propensity score matching by a 1:2 Greedy algorithm. Ultrasonography, contrast-enhanced ultrasonography (US/CEUS) and SPECT/CT showed that the co-transplanted bone fragments remained viable for 6 months, subsequently shrank, and finally degenerated 10 months post-transplantation. BF-BM resulted in earlier kidney recovery and more robust long-term kidney function. Throughout 5 years of follow-up, BF-BM had regulatory effects on dendritic cells (DCs), T helper (Th1/Th2) cells and regulatory T cells (Tregs). Both alloantigen-specific lymphocyte proliferation and panel reactive antibody levels were negative in all recipients with or without BF-BM. In addition, the BF-BM group experienced few complications during the 5-year follow-up (as did the donors)-this was not different from the controls. In conclusion, BF-BM is safe and benefits recipients by protecting the kidney and regulating the immune response.
Keywords: bone fragment co-transplantation; bone marrow aspirate infusion; immune regulation; kidney protection; living kidney transplantation (LKT).
Copyright © 2021 Luo, Zhang, Zou, Wang, Chen, Li, Li, Wang, Chen, Ming, Zhu and Gong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

