Total neoadjuvant therapy vs standard therapy of locally advanced rectal cancer with high-risk factors for failure
- PMID: 33643528
- PMCID: PMC7896420
- DOI: 10.4251/wjgo.v13.i2.119
Total neoadjuvant therapy vs standard therapy of locally advanced rectal cancer with high-risk factors for failure
Abstract
Background: For locally advanced rectal cancer (LARC), standard therapy [consisting of neoadjuvant chemoradiotherapy (CRT), surgery, and adjuvant chemotherapy (ChT)] achieves excellent local control. Unfortunately, survival is still poor due to distant metastases, which remains the leading cause of death among these patients. In recent years, the concept of total neoadjuvant treatment (TNT) has been developed, whereby all systemic ChT-mainly affecting micrometastases-is applied prior to surgery.
Aim: To compare standard therapy and total neoadjuvant therapy for LARC patients with high-risk factors for failure.
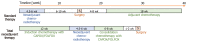
Methods: In a retrospective study, we compared LARC patients with high-risk factors for failure who were treated with standard therapy or with TNT. High-risk for failure was defined according to the presence of at least one of the following factors: T4 stage; N2 stage; positive mesorectal fascia; extramural vascular invasion; positive lateral lymph node. TNT consisted of 12 wk of induction ChT with capecitabine and oxaliplatin or folinic acid, fluorouracil and oxaliplatin, CRT with capecitabine, and 6-8 wk of consolidation ChT with capecitabine and oxaliplatin or folinic acid, fluorouracil and oxaliplatin prior to surgery. The primary endpoint was pathological complete response (pCR). In total, 72 patients treated with standard therapy and 89 patients treated with TNT were included in the analysis.
Results: Compared to standard therapy, TNT showed a higher proportion of pCR (23% vs 7%; P = 0.01), a lower neoadjuvant rectal score (median: 8.43 vs 14.98; P < 0.05), higher T-and N-downstaging (70% and 94% vs 51% and 86%), equivalent R0 resection (95% vs 93%), shorter time to stoma closure (mean: 20 vs 33 wk; P < 0.05), higher compliance during systemic ChT (completed all cycles 87% vs 76%; P < 0.05), lower proportion of acute toxicity grade ≥ 3 during ChT (3% vs 14%, P < 0.05), and equivalent acute toxicity and compliance during CRT and in the postoperative period. The pCR rate in patients treated with TNT was significantly higher in patients irradiated with intensity-modulated radiotherapy/volumetric-modulated arc radiotherapy than with 3D conformal radiotherapy (32% vs 9%; P < 0.05).
Conclusion: Compared to standard therapy, TNT provides better outcome for LARC patients with high-risk factors for failure, in terms of pCR and neoadjuvant rectal score.
Keywords: Locally advanced rectal cancer; Neoadjuvant rectal cancer score; Pathological complete response; Total neoadjuvant therapy.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no financial relationships to disclose.
Figures
References
-
- Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, Gelderblom H, Kapiteijn E, Leer JWH, Marijnen CAM, Martijn H, Meershoek-Klein Kranenbarg E, Nagtegaal ID, Påhlman L, Punt CJA, Putter H, Roodvoets AGH, Rutten HJT, Steup WH, Glimelius B, van de Velde CJH. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo) radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701. - PubMed
-
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. - PubMed
-
- Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, Tebbutt N, Hill M, Ross PJ, Massey A, Oates J. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–674. - PubMed
-
- Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, Saigi E, Estevan R, Mira M, Polo S, Hernandez A, Gallen M, Arias F, Serra J, Alonso V. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–865. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials