Role of the mesenchymal stem cells derived from adipose tissue in changing the rate of breast cancer cell proliferation and autophagy, in vitro and in vivo
- PMID: 33643577
- PMCID: PMC7894629
- DOI: 10.22038/ijbms.2020.51461.11678
Role of the mesenchymal stem cells derived from adipose tissue in changing the rate of breast cancer cell proliferation and autophagy, in vitro and in vivo
Abstract
Objectives: Autophagy is an intracellular degradation system of damaged proteins and organelles; however, the role of autophagy in the progression of cancer remains unclear. In recent years, mesenchymal stem cell (MSC)-based approaches have attracted considerable attention for anti-cancer therapy. The present study aimed to examine the interaction of MSCs with the breast cancer cells under autophagy-induced conditions.
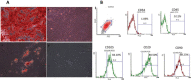
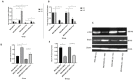
Materials and methods: In this study, MSCs isolated from human adipose tissue were co-cultured with MDA-MB 231, a breast cancer cell line, and the autophagy process was induced by tunicamycin treatment. The cell viability was monitored by the MTT assay, and the cells were recovered at different time intervals (24 or 48 hours) to determine autophagy markers such as Beclin, mTOR and the ratio of LC3II/I expression. Additionally, the animal study was conducted using a mouse model of breast cancer treated with isogenic adipose-derived MSCs, and the expression of Beclin and Ki67 was determined using immunohistochemistry in breast tumor tissue.
Results: In cancer cells co-cultured with MSCs, the cell proliferation was increased, the Beclin expression and the LC3II/I protein ratio were decreased, and the mTOR expression was increased in MDA-MB 231 upon co-cultured with MSCs. Direct injection of MSCs to a mouse model of breast cancer showed an increase in tumor volume, an increase in the accumulation of Ki67 and a decrease in the Beclin expression in tumor tissues.
Conclusion: The data may suggest that suppressed autophagy in breast cancer cells is probably a mechanism by which MSCs can induce cancer cell proliferation.
Keywords: Biomarker; Carcinoma; Cell growth; Cell therapy; Molecular pathway.
Figures





References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. - PubMed
-
- Timaner M, Tsai K, Shaked Y, editors The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2019 Elsevier. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
