Changes in the extracellular microenvironment and osteogenic responses of mesenchymal stem/stromal cells induced by in vitro direct electrical stimulation
- PMID: 33643602
- PMCID: PMC7894594
- DOI: 10.1177/2041731420974147
Changes in the extracellular microenvironment and osteogenic responses of mesenchymal stem/stromal cells induced by in vitro direct electrical stimulation
Abstract
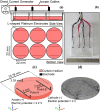
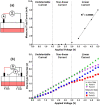
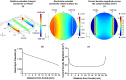
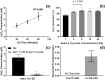
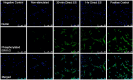
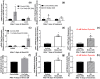
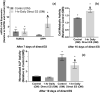
Electrical stimulation (ES) has potential to be an effective tool for bone injury treatment in clinics. However, the therapeutic mechanism associated with ES is still being discussed. This study aims to investigate the initial mechanism of action by characterising the physical and chemical changes in the extracellular environment during ES and correlate them with the responses of mesenchymal stem/stromal cells (MSCs). Computational modelling was used to estimate the electrical potentials relative to the cathode and the current density across the cell monolayer. We showed expression of phosphorylated ERK1/2, c-FOS, c-JUN, and SPP1 mRNAs, as well as the increased metabolic activities of MSCs at different time points. Moreover, the average of 2.5 μM of H2O2 and 34 μg/L of dissolved Pt were measured from the electrically stimulated media (ES media), which also corresponded with the increases in SPP1 mRNA expression and cell metabolic activities. The addition of sodium pyruvate to the ES media as an antioxidant did not alter the SPP1 mRNA expression, but eliminated an increase in cell metabolic activities induced by ES media treatment. These findings suggest that H2O2 was influencing cell metabolic activity, whereas SPP1 mRNA expression was regulated by other faradic by-products. This study reveals how different electrical stimulation regime alters cellular regenerative responses and the roles of faradic by-products, that might be used as a physical tool to guide and control cell behaviour.
Keywords: Electrical stimulation; bone regeneration; computational modelling; faradic by-products; mesenchymal stem/stromal cells.
© The Author(s) 2021.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Yasuda I. The classic fundamental aspects of fracture treatment. Clin Orthop Relat R 1977; 124: 5–8. - PubMed
-
- Bassett CA, Pawluk RJ, Becker RO. Effects of electric currents on bone in vivo. Nature 1964; 204: 652–654. - PubMed
-
- Baranowski TJ, Black J, Brighton CT, et al. Electrical osteogenesis by low direct current. J Orthop Res 1983; 1: 120–128. - PubMed
-
- Brighton CT, Friedenberg ZB, Black J, et al. Electrically induced osteogenesis - relationship between charge, current-density, and the amount of bone formed - introduction of a new cathode concept. Clin Orthop Relat R 1981; 161: 122–132. - PubMed
-
- Bassett CA, Pawluk RJ, Pilla AA. Acceleration of fracture repair by electromagnetic fields. A surgically noninvasive method. Ann N Y Acad Sci 1974; 238: 242–262. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

