Proteinaceous Hydrogels for Bioengineering Advanced 3D Tumor Models
- PMID: 33643799
- PMCID: PMC7887602
- DOI: 10.1002/advs.202003129
Proteinaceous Hydrogels for Bioengineering Advanced 3D Tumor Models
Abstract
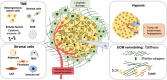
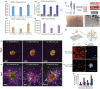
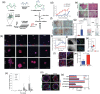
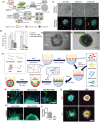
The establishment of tumor microenvironment using biomimetic in vitro models that recapitulate key tumor hallmarks including the tumor supporting extracellular matrix (ECM) is in high demand for accelerating the discovery and preclinical validation of more effective anticancer therapeutics. To date, ECM-mimetic hydrogels have been widely explored for 3D in vitro disease modeling owing to their bioactive properties that can be further adapted to the biochemical and biophysical properties of native tumors. Gathering on this momentum, herein the current landscape of intrinsically bioactive protein and peptide hydrogels that have been employed for 3D tumor modeling are discussed. Initially, the importance of recreating such microenvironment and the main considerations for generating ECM-mimetic 3D hydrogel in vitro tumor models are showcased. A comprehensive discussion focusing protein, peptide, or hybrid ECM-mimetic platforms employed for modeling cancer cells/stroma cross-talk and for the preclinical evaluation of candidate anticancer therapies is also provided. Further development of tumor-tunable, proteinaceous or peptide 3D microtesting platforms with microenvironment-specific biophysical and biomolecular cues will contribute to better mimic the in vivo scenario, and improve the predictability of preclinical screening of generalized or personalized therapeutics.
Keywords: 3D in vitro models; cancers; hydrogels; peptides; proteins.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
