P. aeruginosa Mediated Necroptosis in Mouse Tumor Cells Induces Long-Lasting Systemic Antitumor Immunity
- PMID: 33643911
- PMCID: PMC7908819
- DOI: 10.3389/fonc.2020.610651
P. aeruginosa Mediated Necroptosis in Mouse Tumor Cells Induces Long-Lasting Systemic Antitumor Immunity
Abstract
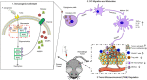
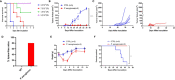
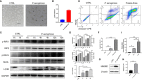
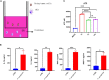
Necroptosis is a form of programmed cell death (PCD) characterized by RIP3 mediated MLKL activation and increased membrane permeability via MLKL oligomerization. Tumor cell immunogenic cell death (ICD) has been considered to be essential for the anti-tumor response, which is associated with DC recruitment, activation, and maturation. In this study, we found that P. aeruginosa showed its potential to suppress tumor growth and enable long-lasting anti-tumor immunity in vivo. What's more, phosphorylation- RIP3 and MLKL activation induced by P. aeruginosa infection resulted in tumor cell necrotic cell death and HMGB1 production, indicating that P. aeruginosa can cause immunogenic cell death. The necrotic cell death can further drive a robust anti-tumor response via promoting tumor cell death, inhibiting tumor cell proliferation, and modulating systemic immune responses and local immune microenvironment in tumor. Moreover, dying tumor cells killed by P. aeruginosa can catalyze DC maturation, which enhanced the antigen-presenting ability of DC cells. These findings demonstrate that P. aeruginosa can induce immunogenic cell death and trigger a robust long-lasting anti-tumor response along with reshaping tumor microenvironment.
Keywords: P. aeruginosa; antitumor immunity; dying tumor cell; necroptosis; tumor microenvironment.
Copyright © 2021 Qi, He, Jin, Yang, Bai, Liu and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy.Oncoimmunology. 2016 Mar 10;5(6):e1149673. doi: 10.1080/2162402X.2016.1149673. eCollection 2016 Jun. Oncoimmunology. 2016. PMID: 27471616 Free PMC article.
-
RIP1, RIP3, and MLKL Contribute to Cell Death Caused by Clostridium perfringens Enterotoxin.mBio. 2019 Dec 17;10(6):e02985-19. doi: 10.1128/mBio.02985-19. mBio. 2019. PMID: 31848291 Free PMC article.
-
Recombinant viruses delivering the necroptosis mediator MLKL induce a potent antitumor immunity in mice.Oncoimmunology. 2020 Aug 20;9(1):1802968. doi: 10.1080/2162402X.2020.1802968. Oncoimmunology. 2020. PMID: 32923163 Free PMC article.
-
Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments.Cell Death Differ. 2014 Jan;21(1):39-49. doi: 10.1038/cdd.2013.84. Epub 2013 Jul 5. Cell Death Differ. 2014. PMID: 23832118 Free PMC article. Review.
-
The role of necroptosis in cancer biology and therapy.Mol Cancer. 2019 May 23;18(1):100. doi: 10.1186/s12943-019-1029-8. Mol Cancer. 2019. PMID: 31122251 Free PMC article. Review.
Cited by
-
Necroptosis Contributes to Persistent Inflammation During Acute Leptospirosis.Front Immunol. 2022 Mar 22;13:810834. doi: 10.3389/fimmu.2022.810834. eCollection 2022. Front Immunol. 2022. PMID: 35392072 Free PMC article.
-
Recent Advances in Bacteria-Based Cancer Treatment.Cancers (Basel). 2022 Oct 9;14(19):4945. doi: 10.3390/cancers14194945. Cancers (Basel). 2022. PMID: 36230868 Free PMC article. Review.
-
Necroptosis pathways in tumorigenesis.Semin Cancer Biol. 2022 Nov;86(Pt 3):32-40. doi: 10.1016/j.semcancer.2022.07.007. Epub 2022 Jul 28. Semin Cancer Biol. 2022. PMID: 35908574 Free PMC article. Review.
-
Oncolytic bacteria: A revolutionary approach to cancer therapy.Open Life Sci. 2025 Jun 10;20(1):20251076. doi: 10.1515/biol-2025-1076. eCollection 2025. Open Life Sci. 2025. PMID: 40519767 Free PMC article. Review.
-
Lung microbiome alterations correlate with immune imbalance in non-small cell lung cancer.Front Immunol. 2025 May 14;16:1589843. doi: 10.3389/fimmu.2025.1589843. eCollection 2025. Front Immunol. 2025. PMID: 40391224 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

