Sex-Specific Survival, Growth, Immunity and Organ Development in Preterm Pigs as Models for Immature Newborns
- PMID: 33643975
- PMCID: PMC7905020
- DOI: 10.3389/fped.2021.626101
Sex-Specific Survival, Growth, Immunity and Organ Development in Preterm Pigs as Models for Immature Newborns
Abstract
Background: After very preterm birth, male infants show higher mortality than females, with higher incidence of lung immaturity, neurological deficits, infections, and growth failure. In modern pig production, piglets dying in the perinatal period (up to 20%) often show signs of immature organs, but sex-specific effects are not clear. Using preterm pigs as model for immature infants and piglets, we hypothesized that neonatal survival and initial growth and immune development depend on sex. Methods: Using data from a series of previous intervention trials with similar delivery and rearing procedures, we established three cohorts of preterm pigs (90% gestation), reared for 5, 9, or 19 days before sample collection (total n = 1,938 piglets from 109 litters). Partly overlapping endpoints among experiments allowed for multiple comparisons between males and females for data on mortality, body and organ growth, gut, immunity, and brain function. Results: Within the first 2 days, males showed higher mortality than females (18 vs. 8%, P < 0.001), but less severe immune response to gram-positive infection. No effect of sex was observed for thermoregulation or plasma cortisol. Later, infection resistance did not differ between sexes, but growth rate was reduced for body (up to -40%) and kidneys (-6%) in males, with higher leucocyte counts (+15%) and lower CD4 T cell fraction (-5%) on day 9 and lower monocyte counts (-18%, day 19, all P < 0.05). Gut structure, function and necrotizing enterocolitis (NEC) incidence were similar between groups, but intestinal weight (-3%) and brush-border enzyme activities were reduced at day 5 (lactase, DPP IV, -8%) in males. Remaining values for blood biochemistry, hematology, bone density, regional brain weights, and visual memory (tested in a T maze) were similar. Conclusion: Following preterm birth, male pigs show higher mortality and slower growth than females, despite limited differences in organ growth, gut, immune, and brain functions. Neonatal intensive care procedures may be particularly important for compromised newborns of the male sex. Preterm pigs can serve as good models to study the interactions of sex- and maturation-specific survival and physiological adaptation in mammals.
Keywords: animal model; cohort; gender; immune; preterm; sex.
Copyright © 2021 Bæk, Cilieborg, Nguyen, Bering, Thymann and Sangild.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



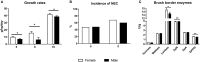
References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. 10.1016/S0140-6736(12)61728-0 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

