All-Polymer Printed Low-Cost Regenerative Nerve Cuff Electrodes
- PMID: 33644015
- PMCID: PMC7902501
- DOI: 10.3389/fbioe.2021.615218
All-Polymer Printed Low-Cost Regenerative Nerve Cuff Electrodes
Abstract
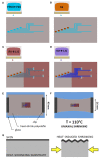
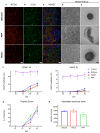
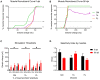
Neural regeneration after lesions is still limited by several factors and new technologies are developed to address this issue. Here, we present and test in animal models a new regenerative nerve cuff electrode (RnCE). It is based on a novel low-cost fabrication strategy, called "Print and Shrink", which combines the inkjet printing of a conducting polymer with a heat-shrinkable polymer substrate for the development of a bioelectronic interface. This method allows to produce miniaturized regenerative cuff electrodes without the use of cleanroom facilities and vacuum based deposition methods, thus highly reducing the production costs. To fully proof the electrodes performance in vivo we assessed functional recovery and adequacy to support axonal regeneration after section of rat sciatic nerves and repair with RnCE. We investigated the possibility to stimulate the nerve to activate different muscles, both in acute and chronic scenarios. Three months after implantation, RnCEs were able to stimulate regenerated motor axons and induce a muscular response. The capability to produce fully-transparent nerve interfaces provided with polymeric microelectrodes through a cost-effective manufacturing process is an unexplored approach in neuroprosthesis field. Our findings pave the way to the development of new and more usable technologies for nerve regeneration and neuromodulation.
Keywords: PEDOT:PSS; inkjet printing; low-cost fabrication; organic bioelectronics; peripheral nerve interfaces; regenerative cuff electrodes; wrinkling.
Copyright © 2021 Ferrari, Rodríguez-Meana, Bonisoli, Cutrone, Micera, Navarro, Greco and del Valle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Asplund M., Nyberg T., Inganäs O. (2010). Electroactive polymers for neural interfaces. Polym. Chem. 1, 1374–1391. 10.1039/c0py00077a - DOI
-
- Badia J., Boretius T., Andreu D., Azevedo-Coste C., Stieglitz T., Navarro X. (2011). Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. J. Neural Eng. 8:036023. 10.1088/1741-2560/8/3/036023 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

