Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications
- PMID: 33644016
- PMCID: PMC7907607
- DOI: 10.3389/fbioe.2021.621748
Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications
Abstract
Mesenchymal stem/stromal cell (MSC) exist within their in vivo niches as part of heterogeneous cell populations, exhibiting variable stemness potential and supportive functionalities. Conventional extensive 2D in vitro MSC expansion, aimed at obtaining clinically relevant therapeutic cell numbers, results in detrimental effects on both cellular characteristics (e.g., phenotypic changes and senescence) and functions (e.g., differentiation capacity and immunomodulatory effects). These deleterious effects, added to the inherent inter-donor variability, negatively affect the standardization and reproducibility of MSC therapeutic potential. The resulting manufacturing challenges that drive the qualitative variability of MSC-based products is evident in various clinical trials where MSC therapeutic efficacy is moderate or, in some cases, totally insufficient. To circumvent these limitations, various in vitro/ex vivo techniques have been applied to manufacturing protocols to induce specific features, attributes, and functions in expanding cells. Exposure to inflammatory cues (cell priming) is one of them, however, with untoward effects such as transient expression of HLA-DR preventing allogeneic therapeutic schemes. MSC functionalization can be also achieved by in vitro 3D culturing techniques, in an effort to more closely recapitulate the in vivo MSC niche. The resulting spheroid structures provide spatial cell organization with increased cell-cell interactions, stable, or even enhanced phenotypic profiles, and increased trophic and immunomodulatory functionalities. In that context, MSC 3D spheroids have shown enhanced "medicinal signaling" activities and increased homing and survival capacities upon transplantation in vivo. Importantly, MSC spheroids have been applied in various preclinical animal models including wound healing, bone and osteochondral defects, and cardiovascular diseases showing safety and efficacy in vivo. Therefore, the incorporation of 3D MSC culturing approach into cell-based therapy would significantly impact the field, as more reproducible clinical outcomes may be achieved without requiring ex vivo stimulatory regimes. In the present review, we discuss the MSC functionalization in 3D settings and how this strategy can contribute to an improved MSC-based product for safer and more effective therapeutic applications.
Keywords: MSC anti-inflammatory properties; MSC functionalization; MSC spheroids; MSC therapeutic properties; mesenchymal stem cell manufacturing.
Copyright © 2021 Kouroupis and Correa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

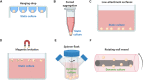
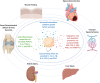
References
-
- Aggarwal S., Pittenger M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105 1815–1822. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

