Senescent Mesenchymal Stem Cells in Myelodysplastic Syndrome: Functional Alterations, Molecular Mechanisms, and Therapeutic Strategies
- PMID: 33644035
- PMCID: PMC7905046
- DOI: 10.3389/fcell.2020.617466
Senescent Mesenchymal Stem Cells in Myelodysplastic Syndrome: Functional Alterations, Molecular Mechanisms, and Therapeutic Strategies
Abstract
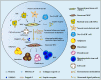
Myelodysplastic syndrome (MDS) is a group of clonal hematopoietic disorders related to hematopoietic stem and progenitor cell dysfunction. However, therapies that are currently used to target hematopoietic stem cells are not effective. These therapies are able to slow the evolution toward acute myeloid leukemia but cannot eradicate the disease. Mesenchymal stem cells (MSCs) have been identified as one of the main cellular components of the bone marrow microenvironment, which plays an indispensable role in normal hematopoiesis. When functional and regenerative capacities of aging MSCs are diminished, some enter replicative senescence, which promotes inflammation and disease progression. Recent studies that investigated the contribution of bone marrow microenvironment and MSCs to the initiation and progression of the disease have offered new insights into the MDS. This review presents the latest updates on the role of MSCs in the MDS and discusses potential targets for the treatment of MDS.
Keywords: bone marrow microenvironment; mesenchymal stem cells; myelodysplastic syndrome; senescence; treatment.
Copyright © 2021 Chen, Li, Weng and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alessandrino E. P., Della Porta M. G., Bacigalupo A., Van Lint M. T., Falda M., Onida F., et al. . (2008). WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood 112, 895–902. 10.1182/blood-2008-03-143735 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

