Extracellular Matrix Patches for Endarterectomy Repair
- PMID: 33644135
- PMCID: PMC7904872
- DOI: 10.3389/fcvm.2021.631750
Extracellular Matrix Patches for Endarterectomy Repair
Abstract
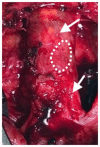
Patch repair is the preferred method for arteriotomy closure following femoral or carotid endarterectomy. Choosing among available patch options remains a clinical challenge, as current evidence suggests roughly comparable outcomes between autologous grafts and synthetic and biologic materials. Biologic patches have potential advantages over other materials, including reduced risk for infection, mitigation of an excessive foreign body response, and the potential to remodel into healthy, vascularized tissue. Here we review the use of decellularized extracellular matrix (ECM) for cardiovascular applications, particularly endarterectomy repair, and the capacity of these materials to remodel into native, site-appropriate tissues. Also presented are data from two post-market observational studies of patients undergoing iliofemoral and carotid endarterectomy patch repair as well as one histologic case report in a challenging iliofemoral endarterectomy repair, all with the use of small intestine submucosa (SIS)-ECM. In alignment with previously reported studies, high patency was maintained, and adverse event rates were comparable to previously reported rates of patch angioplasty. Histologic analysis from one case identified constructive remodeling of the SIS-ECM, consistent with the histologic characteristics of the endarterectomized vessel. These clinical and histologic results align with the biologic potential described in the academic ECM literature. To our knowledge, this is the first histologic demonstration of SIS-ECM remodeling into site-appropriate vascular tissues following endarterectomy. Together, these findings support the safety and efficacy of SIS-ECM for patch repair of femoral and carotid arteriotomy.
Keywords: atherosclerosis; biologic patch; cerebrovascular disease; endarterectomy; extracellular matrix; peripheral arterial disease; tissue integration; tissue regeneration.
Copyright © 2021 Allen, Adams, Badylak, Garrett, Mouawad, Oweida, Parikshak and Sultan.
Conflict of interest statement
The authors declare that this study received funding from Aziyo Biologics, Inc. The funder had the following involvement with the study: study design, data collection and analysis, and supported preparation of the manuscript.
Figures




References
-
- van Hinsbergh VWM. Physiology of blood vessels. In: Krams R, Bäck M. editors. The ESC Textbook of Vascular Biology Oxford: Oxford University Press; (2017). p. 17–30. 10.1093/med/9780198755777.003.0002 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

