Modification of Natural Proanthocyanidin Oligomers and Polymers Via Chemical Oxidation under Alkaline Conditions
- PMID: 33644580
- PMCID: PMC7906247
- DOI: 10.1021/acsomega.0c05515
Modification of Natural Proanthocyanidin Oligomers and Polymers Via Chemical Oxidation under Alkaline Conditions
Abstract
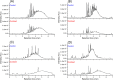
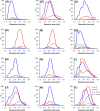
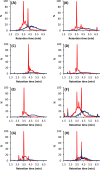
We tested the susceptibility of 102 proanthocyanidin (PA)-rich plant extracts to oxidation under alkaline conditions and the possibility to produce chemically modified PAs via oxidation. Both the nonoxidized and the oxidized extracts were analyzed using group-specific ultrahigh-performance liquid chromatography-diode array detection-tandem mass spectrometry (UHPLC-DAD-MS/MS) methods capable of detecting procyanidin (PC) and prodelphinidin (PD) moieties along the two-dimensional (2D) chromatographic fingerprints of plant PAs. The results indicated different reactivities for PCs and PDs. When detected by UHPLC-DAD only, most of the PC-rich samples exhibited only a subtle change in their PA content, but the UHPLC-MS/MS quantitation showed that the decrease in the PC content varied by 0-100%. The main reaction route was concluded to be intramolecular. The PD-rich and galloylated PAs showed a different pattern with high reductions in the original PA content by both ultraviolet (UV) and MS/MS quantitation, accompanied by the shifted retention times of the chromatographic PA humps. In these samples, both intra- and intermolecular reactions were indicated.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Characterization of Natural and Alkaline-Oxidized Proanthocyanidins in Plant Extracts by Ultrahigh-Resolution UHPLC-MS/MS.Molecules. 2021 Mar 26;26(7):1873. doi: 10.3390/molecules26071873. Molecules. 2021. PMID: 33810382 Free PMC article.
-
Isolation of chemically well-defined semipreparative liquid chromatography fractions from complex mixtures of proanthocyanidin oligomers and polymers.J Chromatogr A. 2018 Nov 16;1576:67-79. doi: 10.1016/j.chroma.2018.09.034. Epub 2018 Sep 26. J Chromatogr A. 2018. PMID: 30314685
-
Alkaline oxidization can increase the in vitro antiparasitic activity of proanthocyanidin-rich plant extracts against Ascarissuum.Exp Parasitol. 2023 May;248:108493. doi: 10.1016/j.exppara.2023.108493. Epub 2023 Mar 6. Exp Parasitol. 2023. PMID: 36889503
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010-2019).Phytochem Anal. 2020 Jul;31(4):413-457. doi: 10.1002/pca.2906. Epub 2019 Dec 17. Phytochem Anal. 2020. PMID: 31849137 Review.
Cited by
-
Functional Fruit Snacks Enriched with Natural Sources of Fructooligosaccharides: Composition, Bioactive Compounds, Biological Activity, and Consumer Acceptance.Molecules. 2025 Jun 7;30(12):2507. doi: 10.3390/molecules30122507. Molecules. 2025. PMID: 40572473 Free PMC article.
-
High intraspecific variability and previous experience affect polyphenol metabolism in polyphagous Lymantria mathura caterpillars.Ecol Evol. 2024 Feb 9;14(2):e10973. doi: 10.1002/ece3.10973. eCollection 2024 Feb. Ecol Evol. 2024. PMID: 38343568 Free PMC article.
-
Affinity of Tannins to Cellulose: A Chromatographic Tool for Revealing Structure-Activity Patterns.Molecules. 2023 Jul 13;28(14):5370. doi: 10.3390/molecules28145370. Molecules. 2023. PMID: 37513244 Free PMC article.
-
Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization.Plants (Basel). 2022 Jun 9;11(12):1544. doi: 10.3390/plants11121544. Plants (Basel). 2022. PMID: 35736694 Free PMC article.
-
A Food-Grade Method for Enhancing the Levels of Low Molecular Weight Proanthocyanidins with Potentially High Intestinal Bioavailability.Int J Mol Sci. 2022 Nov 4;23(21):13557. doi: 10.3390/ijms232113557. Int J Mol Sci. 2022. PMID: 36362344 Free PMC article.
References
-
- Porter L.J.Condensed tannins. In Natural products of woody plants I; Rowe J.W., Ed. Springer-Verlag, Berlin, Germany, 1989, 651–690.
-
- Ferreira D.; Slade D.; Marais P.J.. Flavans and proanthocyanidins. In Flavonoids: Chemistry, biochemistry and applications. Andersen O.M., Markham K.R., Eds.; Taylor and Francis, New York, US, 2005, 553–616.
-
- Haslam E. Symmetry and promiscuity in procyanidin biochemistry. Phytochemistry 1977, 16, 1625–1640. 10.1016/0031-9422(71)85060-4. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

