Polymerization of Biobased Farnesene in Miniemulsions by Nitroxide-Mediated Polymerization
- PMID: 33644601
- PMCID: PMC7905948
- DOI: 10.1021/acsomega.0c05992
Polymerization of Biobased Farnesene in Miniemulsions by Nitroxide-Mediated Polymerization
Abstract
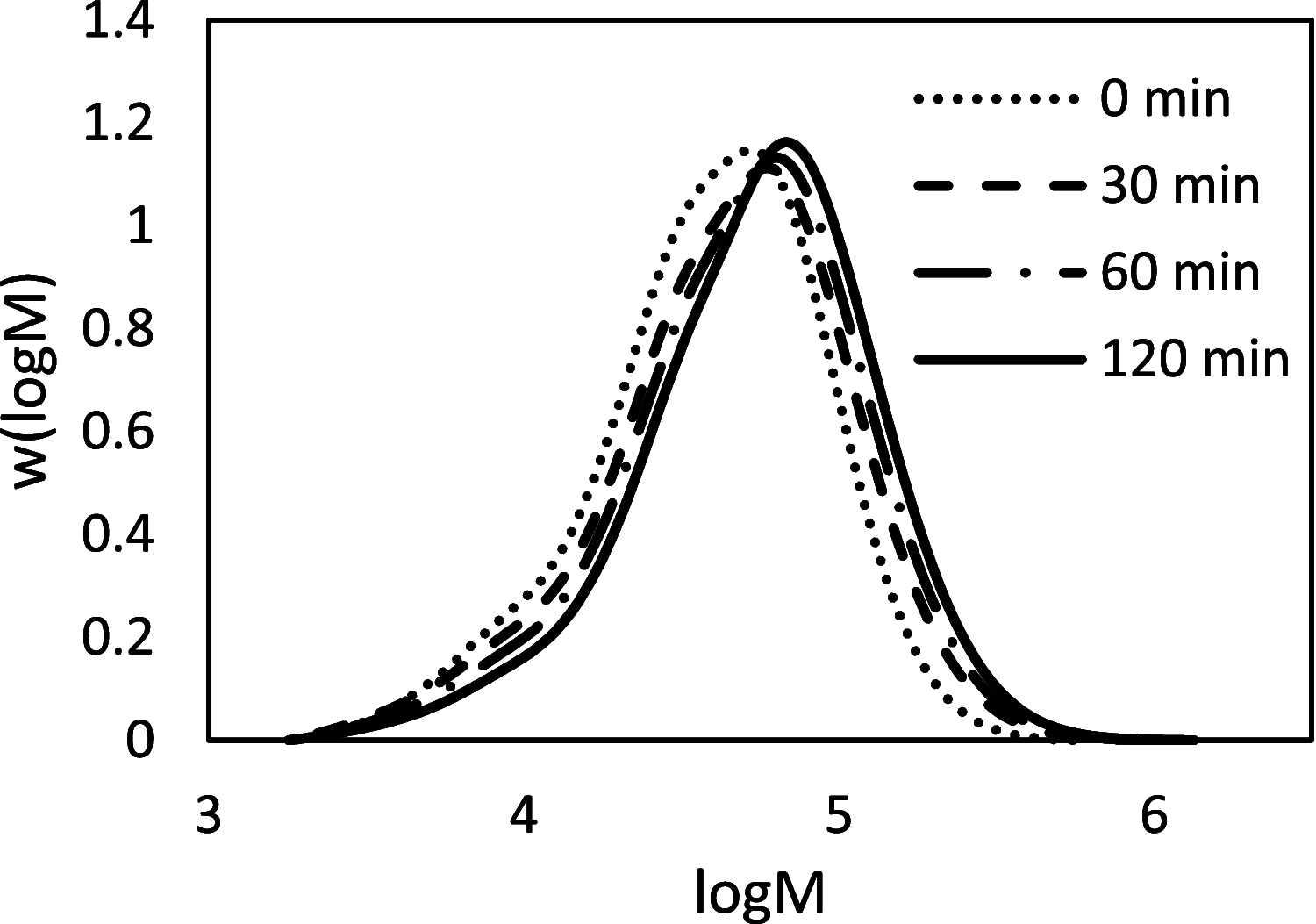
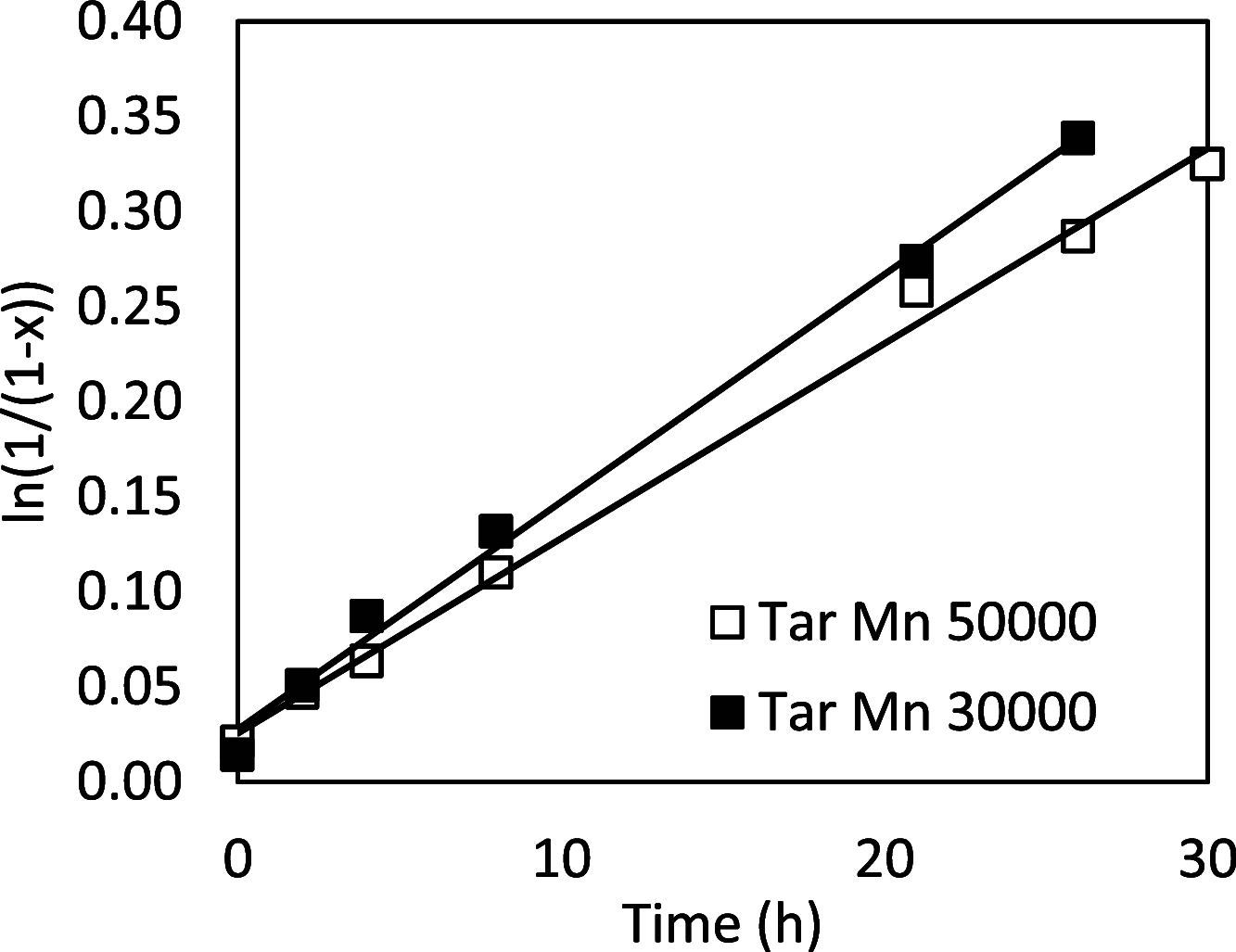
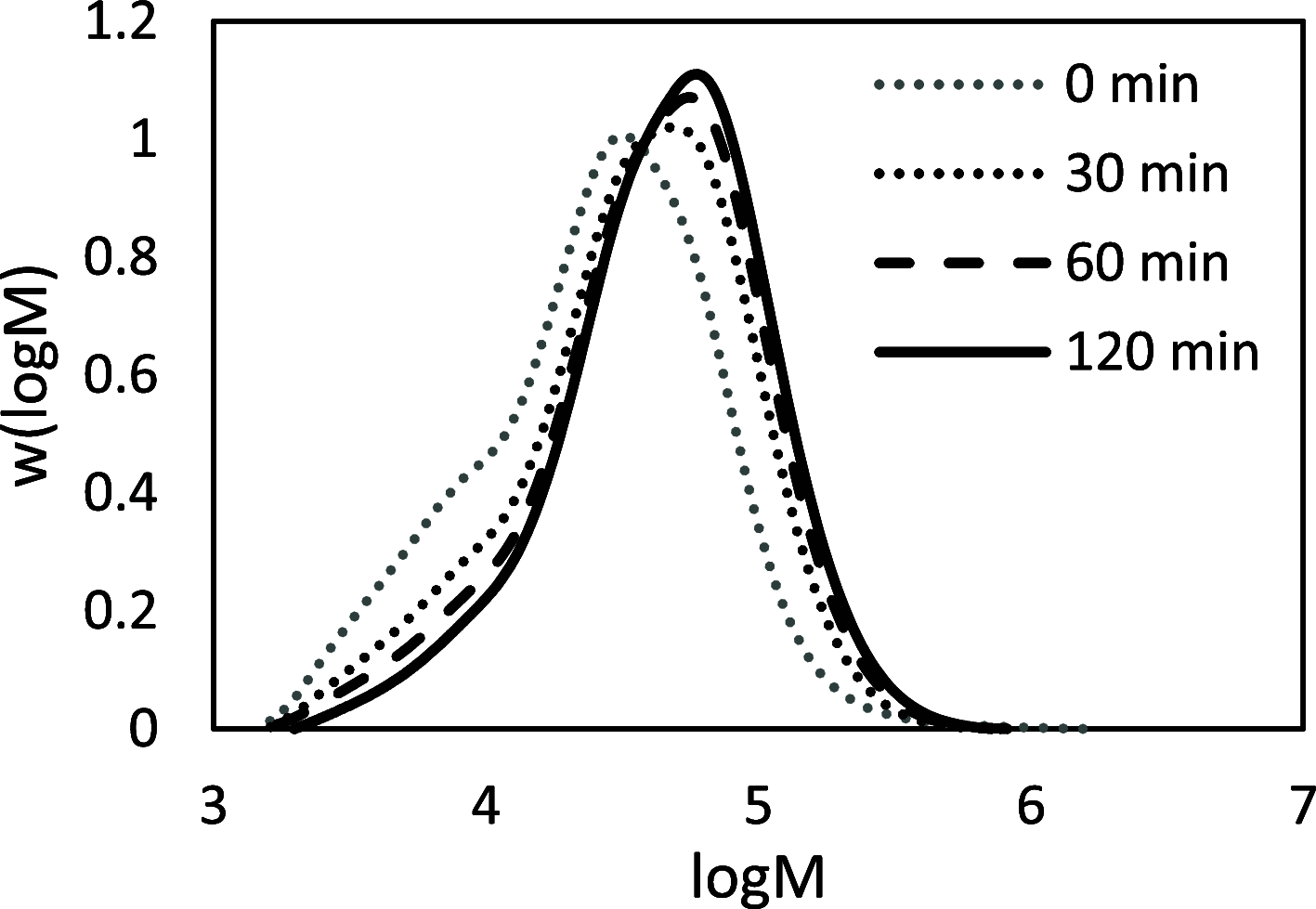
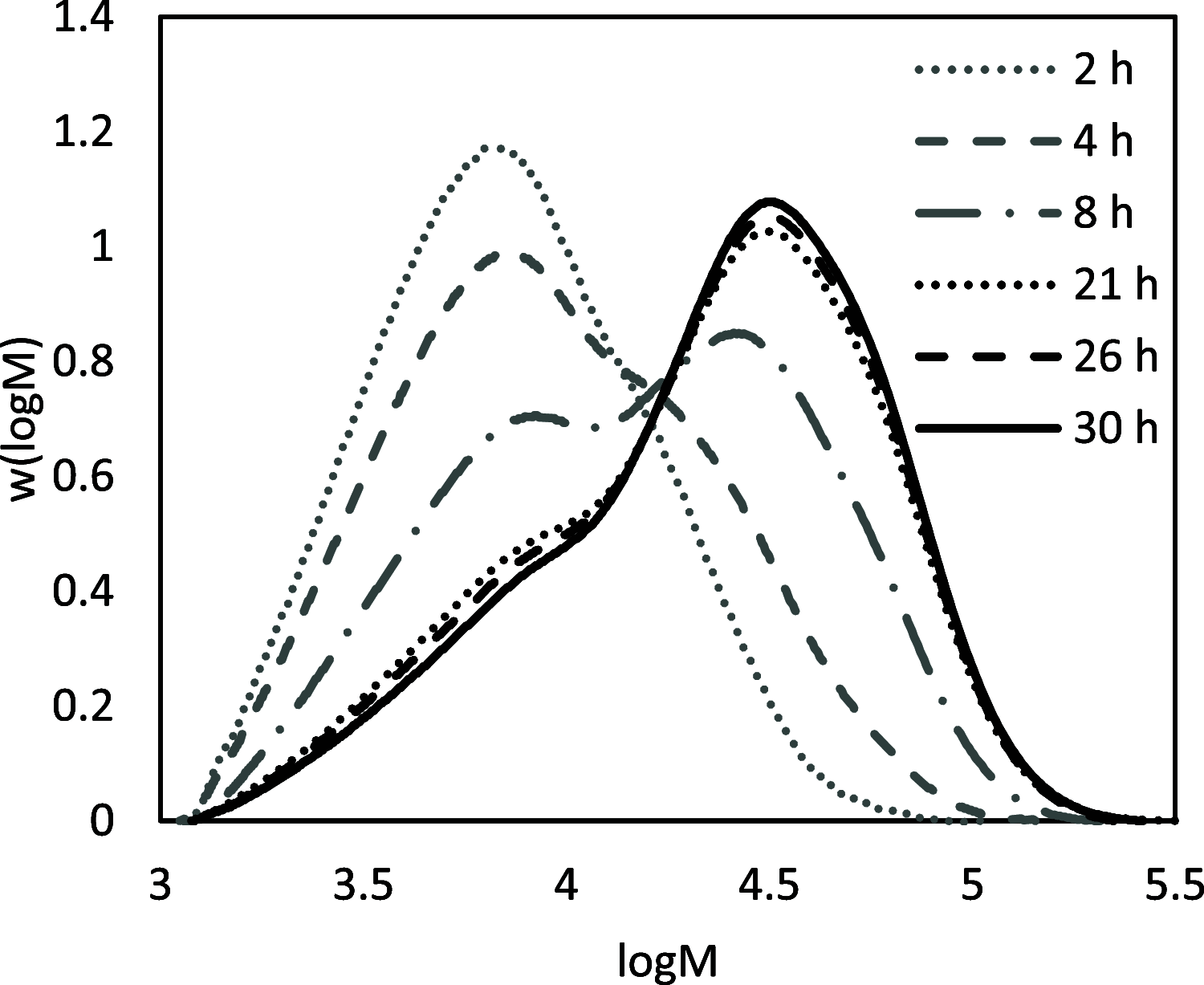
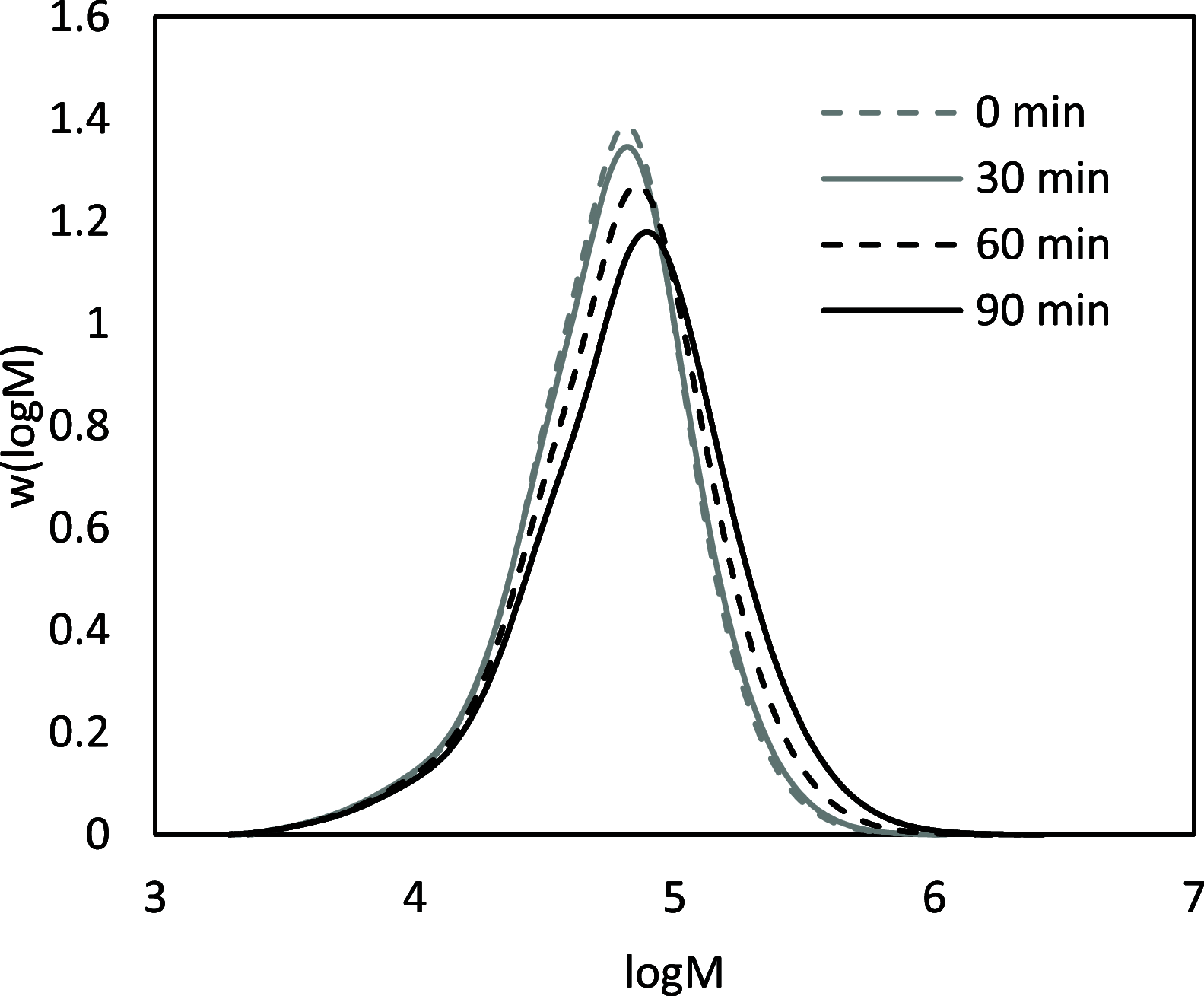
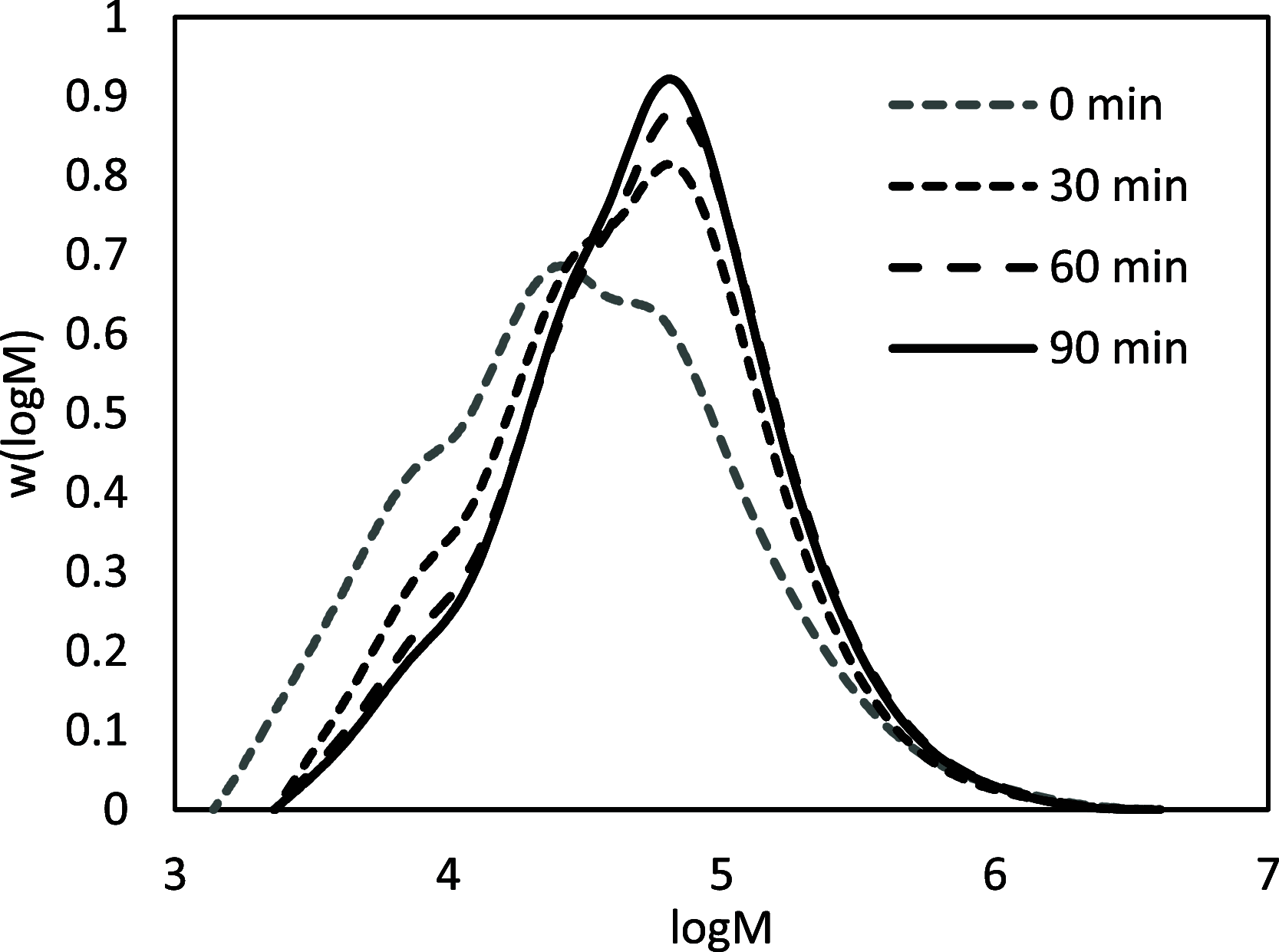
Biobased farnesene (Far) was polymerized by nitroxide-mediated polymerization in miniemulsions using two different alkoxyamine initiators, the SG1-based and succinimidyl-modified BlocBuilder (NHS-BB) and Dispolreg 007 (D7). Stable emulsions were observed after 30 h of reaction at 90 °C, where NHS-BB-initiated systems resulted in smaller particles (∼300 nm) than using D7 (∼400 nm). Successful chain extension of the poly(Far) macroinitiators (24,500-39,700 g mol-1) with styrene were achieved using 15 wt % surfactant relative to monomer concentration. Compartmentalization effects were not observed in these emulsions as the polymerization rate was still much slower compared to the bulk, even though Z-averaged particle sizes were around 300-400 nm. Finally, all biobased diblock copolymers were synthesized by chain-extending poly(Far) macroinitiators with isobornyl methacrylate (iBOMA), where the D7 initiator showed more effective chain extension (less unreacted macroinitiator) than NHS-BB.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Morton M.; McGrath J. E.; Juliano P. C. Structure-property relationships for styrene-diene thermoplastic elastomers. J. Polym. Sci., Part C: Polym. Symp. 1969, 26, 99–115. 10.1002/polc.5070260107. - DOI
-
- Drobny J. G.15-Applications of Thermoplastic Elastomers. In Handbook of Thermoplastic Elastomers; Drobny J. G., Ed.; William Andrew Publishing: Norwich, NY, 2007; pp 281–315.
-
- Wang S.; Wang Q.; Wu X.; Zhang Y. Asphalt modified by thermoplastic elastomer based on recycled rubber. Constr. Build. Mater. 2015, 93, 678–684. 10.1016/j.conbuildmat.2015.06.047. - DOI
-
- Custro S.; Zazzetta A.. Styrene-isoprene block copolymers. U.S. Patent 5,284,915 A, 1994.
LinkOut - more resources
Full Text Sources
Other Literature Sources

