Assessing the impact of COVID-19 on liver cancer management (CERO-19)
- PMID: 33644725
- PMCID: PMC7901294
- DOI: 10.1016/j.jhepr.2021.100260
Assessing the impact of COVID-19 on liver cancer management (CERO-19)
Abstract
Background & aims: The coronavirus disease 2019 (COVID-19) pandemic has posed unprecedented challenges to healthcare systems and it may have heavily impacted patients with liver cancer (LC). Herein, we evaluated whether the schedule of LC screening or procedures has been interrupted or delayed because of the COVID-19 pandemic.
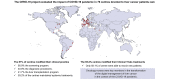
Methods: An international survey evaluated the impact of the COVID-19 pandemic on clinical practice and clinical trials from March 2020 to June 2020, as the first phase of a multicentre, international, and observational project. The focus was on patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma, cared for around the world during the first COVID-19 pandemic wave.
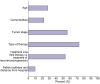
Results: Ninety-one centres expressed interest to participate and 76 were included in the analysis, from Europe, South America, North America, Asia, and Africa (73.7%, 17.1%, 5.3%, 2.6%, and 1.3% per continent, respectively). Eighty-seven percent of the centres modified their clinical practice: 40.8% the diagnostic procedures, 80.9% the screening programme, 50% cancelled curative and/or palliative treatments for LC, and 41.7% modified the liver transplantation programme. Forty-five out of 69 (65.2%) centres in which clinical trials were running modified their treatments in that setting, but 58.1% were able to recruit new patients. The phone call service was modified in 51.4% of centres which had this service before the COVID-19 pandemic (n = 19/37).
Conclusions: The first wave of the COVID-19 pandemic had a tremendous impact on the routine care of patients with liver cancer. Modifications in screening, diagnostic, and treatment algorithms may have significantly impaired the outcome of patients. Ongoing data collection and future analyses will report the benefits and disadvantages of the strategies implemented, aiding future decision-making.
Lay summary: The coronavirus disease 2019 (COVID-19) pandemic has posed unprecedented challenges to healthcare systems globally. Herein, we assessed the impact of the first wave pandemic on patients with liver cancer and found that routine care for these patients has been majorly disrupted, which could have a significant impact on outcomes.
Keywords: BCLC, Barcelona Clinic Liver Cancer; CERO-19, Liver Cancer Outcome in the COVID-19-pandemic Project; COVID-19; COVID-19, coronavirus disease 2019; Cholangiocarcinoma; Clinical trials; ENS-CCA, European Network for the Study of Cholangiocarcinoma; HCC, hepatocellular carcinoma; Hepatocellular carcinoma; LC, liver cancer; LT, liver transplantation; Liver cancer; Management; Nurses; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; iCCA, intrahepatic cholangiocarcinoma.
© 2021 The Authors.
Conflict of interest statement
SM.-M.: Speaker fees from Bayer and travel funding from 10.13039/100004326Bayer and 10.13039/501100014382Eisai. V.S.: Travel grants from 10.13039/100004326Bayer. A.F.: Lecture fees from Bayer, Gilead and MSD; consultancy fees from Bayer, AstraZeneca, Roche and Guerbert. J-C.N.: Received research grant from 10.13039/100004326Bayer for Inserm UMR1138. L.R.: Reports receiving consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, Celgene, Eisai, Exelixis, Hengrui, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi; lectures fees from AbbVie, Amgen, Eisai, Gilead, Incyte, Ipsen, Lilly, Roche, Sanofi; travel fees from Ipsen; and institutional research funding from Agios, ARMO BioSciences, 10.13039/100004325AstraZeneca, BeiGene, 10.13039/501100003769Eisai, 10.13039/100010544Exelixis, 10.13039/100006591Fibrogen, Incyte, 10.13039/501100014382Ipsen, 10.13039/100004312Lilly, 10.13039/100007054MSD, 10.13039/100004337Roche. B.S.: Reports consultancy fees from Adaptimmune, AstraZeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical, Terumo; and research grants from 10.13039/100002491BMS and Sirtex Medical. J. Bruix: Consultancy: AbbVie, ArQule, Astra, Basilea, Bayer, BMS, Daiichi Sankyo, GlaxoSmithKline, Gilead, Kowa, Lilly, Medimune, Novartis, Onxeo, Polaris, Quirem, Roche, Sanofi-Aventis, Sirtex, Terumo/Grants: 10.13039/100004326Bayer and 10.13039/501100014382Ipsen. M.S.Z.: Received speaker fees and travel grants from 10.13039/100004326Bayer and 10.13039/100014869BTG, 10.13039/100007054MSD. M.B.: Consultant and Advisory Board for: Bayer Pharma, Ipsen, BMS, Eisai, Roche, AstraZeneca, Sirtex Medical. D.J.P.: Received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, and AstraZeneca; received research funding (to institution) from MSD and BMS. T.M.: Consultancy: Eisai, Roche, BTG, Ipsen, Bayer, Adaptimmune. Research funding: Bayer, BTG. H.W.: Served as speaker for Bayer, Eisai, and Ipsen, and as a consultant for Bayer, Eisai, Lilly, BMS, Roche, and Ipsen. B.M.: Consultancy: Bayer-Shering Pharma /Speaker fees: Eisai, MSDG. C. Consultancy fees from Bayer, Ipsen. P.R.G.: Bayer, BMS, MSD, AstraZeneca, Adaptimmune, Sirtex, Lilly Ipsen, Roche, Eisai. M.R.A.S.: Has received Research grants, advisory board or speaker fees for 10.13039/100006483AbbVie, 10.13039/100004326Bayer, Biolab, Intercept, 10.13039/501100014382Ipsen, 10.13039/100008799Gilead, 10.13039/100009947MSD, 10.13039/100004336Novartis, and 10.13039/100004337Roche. J.T.: Has received research grants from 10.13039/100004337Roche and 10.13039/501100014382Ipsen. He has received speaker and consulting honoraria from AstraZeneca, Amgen, Bayer Healthcare, Bristol Myers-Squibb, Eisai, Ipsen, Merck Serono, Merck Sharp & Dome, Lilly Imclone, and Roche. J. Bridgewater: Consultancy Bayer, BMS, Incyte, Taiho, Roche, MSD and Merck Serono. Research funding from Incyte. G.C.: Consultancy fees from Bayer, Ipsen. A.L.: Consultancy CAScination, Advisory Board Neuwave and Histosonics. H.T.: Speaker fees from AbbVie, Gilead, MSD, and Bayer. R.V.: Research grant from 10.13039/100006483Abbvie. A.M.M.P.: Speaker honorarium from Bayer, BMS, Boston Scientific and EISAI. Consulting honorarium from Bayer, AstraZeneca and EISAI. Advisory honorarium from Bayer, AstraZeneca and EISAI. Grants from 10.13039/100004326Bayer and 10.13039/100008497Boston Scientific. M.D.: Has received consulting and training fees from Bayer and Eisai. B.B.: Reports Consultancy for GenMab (paid to Institution); Advisory Boards for Roche (paid to Institution), Eisai Europe Limited (paid to Institution), research grant from 10.13039/100006436Celgene Ltd (paid to Institution), Speakers Bureau for Eisai Europe Limited (paid to Institution), Travel and registration for Congress from Bayer. L.d.F.: Lectures fees from BMS, Roche and Bayer. B.M.M.: Advisory/Speaker: Amgen, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Merck, Roche, Sanoffi Genzyme, Taiho. Expert Testimony: Eisai, Roche. Travel: Eisai, Merck. Research: Sillajen (Individual); AstraZeneca, H3/Eisai, Galera, GSK, Exelixis (Institution). M.S.: Travel/ accommodation/meeting expenses: Bayer. Eisai. Speaker fees: Bayer. C.R.L.: Travel grants from 10.13039/100004326Bayer. M.R-G.: Reports grants from Intercept, grants from 10.13039/100005564Gilead-Sciences, personal fees from Shionogi, personal fees from Alfa-Wasserman, personal fees from Prosciento, personal fees from Kaleido, personal fees from Novonrdisk, personal fees from MSD, personal fees from BMS, personal fees from Allergan, personal fees from Boehriger-Ingelheim, personal fees from Zydus, personal fees from Intercept Pharma, personal fees from Gilead-Sciences, outside the submitted work. F.P.: Disclosures: Received speaker honoraria from Bayer, Roche, LKM-Biotoscana, RAFFO. Research Grants from INC Argentinean 10.13039/100013137National Institute of Corrections, 10.13039/100004337Roche. V.M.: Lectures sponsored by Bayer. G.B.: Advisory board Eli-Lilly and Incyte. M. Vergara: Travel grants from 10.13039/100004326Bayer, 10.13039/100008799Gilead, 10.13039/100009947MSD and 10.13039/100006483Abbvie. Lectures sponsored by Gilead, Abbvie, Intercept, and MSD. M.L.: Lectures and educational presentations: Abbvie. Travel/accommodation, meeting expenses covered by Bayer, Gilead, Abbvie. M.I.: Received speaker honoraria from Bayer, Gilead Sciences, BMS, Janssen, Ipsen, MSD, BTG-Boston Scientific, AbbVie, EISAI, and was consultant for BTG-Boston Scientific, Bayer, and Guerbet. M.R.: Consultancy: Bayer-Schering Pharma, BMS, Roche, Ipsen, AstraZeneca, Lilly, BTG/Paid conferences: Bayer-Schering Pharma, BMS, Gilead, Lilly/Research Grants: 10.13039/100004326Bayer-Schering Pharma, 10.13039/501100014382Ipsen. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

