CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity
- PMID: 33644766
- PMCID: PMC7116816
- DOI: 10.1038/s43018-020-00133-0
CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity
Abstract
Intratumoral regulatory T cell (Treg) abundance associates with diminished anti-tumor immunity and poor prognosis in human cancers. Recent work demonstrates that CD25, the high affinity receptor subunit for IL-2, is a selective target for Treg depletion in mouse and human malignancies; however, anti-human CD25 antibodies have failed to deliver clinical responses against solid tumors due to bystander IL-2 receptor signaling blockade on effector T cells, which limits their anti-tumor activity. Here we demonstrate potent single-agent activity of anti-CD25 antibodies optimized to deplete Tregs whilst preserving IL-2-STAT5 signaling on effector T cells, and demonstrate synergy with immune checkpoint blockade in vivo. Pre-clinical evaluation of an anti-human CD25 (RG6292) antibody with equivalent features demonstrates, in both non-human primates and humanized mouse models, efficient Treg depletion with no overt immune-related toxicities. Our data supports the clinical development of RG6292 and evaluation of novel combination therapies incorporating non-IL-2 blocking anti-CD25 antibodies in clinical studies.
Conflict of interest statement
Competing Interests A patent application WO/2018/167104, with relevance to this work has been filed by Cancer Research Technology Limited and Tusk Therapeutics and we want to declare our relationship with this patent. I.S. F.A.V., S.A.Q., K.S.P., A.G., J.S., P.M., are named inventors on these patents. F.A.V., S.A.Q., I.S. and K.S.P. receive royalties related to these patents. S.A.Q. is an advisor to TUSK/Roche. M.A., R.F., J.E., C.M., J.S., B.J., L.L., H.K., J.B., S.B., C.B., E. M-B. and R.S. are employees of Roche, which plan clinical development of the drug. M.A., R.F., J.E., C.M., J.S., B.J., H.K., J.B., S.B., C.B., E. M-B. and R.S. have shares in the companies to which the patents belong.
Figures
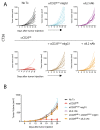


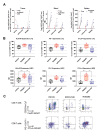

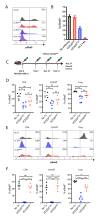
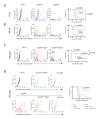


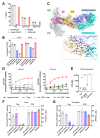
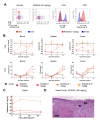
Comment in
-
Anti-CD25 antibody tips the T cell balance.Nat Rev Drug Discov. 2021 Jan;20(1):18. doi: 10.1038/d41573-020-00206-w. Nat Rev Drug Discov. 2021. PMID: 33247220 No abstract available.
-
How to kill Treg cells for immunotherapy.Nat Cancer. 2020 Dec;1(12):1134-1135. doi: 10.1038/s43018-020-00155-8. Nat Cancer. 2020. PMID: 35121936 No abstract available.
References
-
- Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H. CD4 + CD25 + T Regulatory Cells Dominate Multiple Immune Evasion Mechanisms in Early but Not Late Phases of Tumor Development in a B Cell Lymphoma Model. J Immunol. 2007;178:6840–6848. - PubMed
-
- Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. - PubMed
-
- Jones E, et al. Depletion of CD25+ regulatory cells results in suppression of melanomagrowth and induction of autoreactivity in mice. Cancer Immun. 2002 - PubMed
-
- Onizuka S, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

