Selective Prebiotic Synthesis of α-Threofuranosyl Cytidine by Photochemical Anomerization
- PMID: 33644959
- PMCID: PMC8252090
- DOI: 10.1002/anie.202101376
Selective Prebiotic Synthesis of α-Threofuranosyl Cytidine by Photochemical Anomerization
Abstract
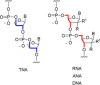
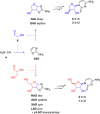
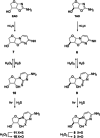
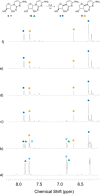
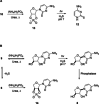
The structure of life's first genetic polymer is a question of intense ongoing debate. The "RNA world theory" suggests RNA was life's first nucleic acid. However, ribonucleotides are complex chemical structures, and simpler nucleic acids, such as threose nucleic acid (TNA), can carry genetic information. In principle, nucleic acids like TNA could have played a vital role in the origins of life. The advent of any genetic polymer in life requires synthesis of its monomers. Here we demonstrate a high-yielding, stereo-, regio- and furanosyl-selective prebiotic synthesis of threo-cytidine 3, an essential component of TNA. Our synthesis uses key intermediates and reactions previously exploited in the prebiotic synthesis of the canonical pyrimidine ribonucleoside cytidine 1. Furthermore, we demonstrate that erythro-specific 2',3'-cyclic phosphate synthesis provides a mechanism to photochemically select TNA cytidine. These results suggest that TNA may have coexisted with RNA during the emergence of life.
Keywords: TNA; nucleotides; origin of life; photochemistry; prebiotic chemistry.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

