Disparate roles of retinoid acid signaling molecules in kidney disease
- PMID: 33645319
- PMCID: PMC8174805
- DOI: 10.1152/ajprenal.00045.2021
Disparate roles of retinoid acid signaling molecules in kidney disease
Abstract
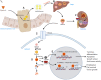
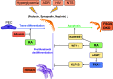
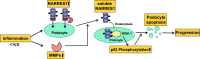
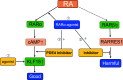
Retinoid acid (RA) is synthesized mainly in the liver and has multiple functions in development, cell differentiation and proliferation, and regulation of inflammation. RA has been used to treat multiple diseases, such as cancer and skin disorders. The kidney is a major organ for RA metabolism, which is altered in the diseased condition. RA is known to have renal-protective effects in multiple animal models of kidney disease. RA has been shown to ameliorate podocyte injury through induction of expression of differentiation markers and regeneration of podocytes from its progenitor cells in animal models of kidney disease. The effects of RA in podocytes are mediated mainly by activation of the cAMP/PKA pathway via RA receptor-α (RARα) and activation of its downstream transcription factor, Kruppel-like factor 15. Screening of RA signaling molecules in human kidney disease has revealed RAR responder protein 1 (RARRES1) as a risk gene for glomerular disease progression. RARRES1, a podocyte-specific growth arrest gene, is regulated by high doses of both RA and TNF-α. Mechanistically, RARRES1 is cleaved by matrix metalloproteinases to generate soluble RARRES1, which then induces podocyte apoptosis through interaction with intracellular RIO kinase 1. Therefore, a high dose of RA may induce podocyte toxicity through upregulation of RARRES1. Based on the current findings, to avoid potential side effects, we propose three strategies to develop future therapies of RA for glomerular disease: 1) develop RARα- and Kruppel-like factor 15-specific agonists, 2) use the combination of a low dose of RAR-α agonist with phosphodiesterase 4 inhibitors, and 3) use a combination of RARα agonist with RARRES1 inhibitors.NEW & NOTEWORTHY Retinoic acid (RA) exerts pleotropic cellular effects, including induction of cell differentiation while inhibiting proliferation and inflammation. These effects are mediated by both RA responsive element-dependent or -independent pathways. In kidneys, RA confers renoprotection by signaling through podocyte RA receptor (RAR)α and activation of cAMP/PKA/Kruppel-like factor 15 pathway to promote podocyte differentiation. Nevertheless, in kidney disease settings, RA can also promote podocyte apoptosis and loss through downstream expression of RAR responder protein 1, a recently described risk factor for glomerular disease progression. These disparate roles of RA underscore the complexity of its effects in kidney homeostasis and disease, and a need to target specific RA-mediated pathways for effective therapeutic treatments against kidney disease progression.
Keywords: glomerular disease; podocytes; retinoic acid; retinoic acid receptor responder protein 1; retinoic acid receptors.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression.J Clin Invest. 2020 Oct 1;130(10):5523-5535. doi: 10.1172/JCI140155. J Clin Invest. 2020. PMID: 32634130 Free PMC article.
-
Novel retinoic acid receptor alpha agonists for treatment of kidney disease.PLoS One. 2011;6(11):e27945. doi: 10.1371/journal.pone.0027945. Epub 2011 Nov 18. PLoS One. 2011. PMID: 22125642 Free PMC article.
-
Retinoic acid improves nephrotoxic serum-induced glomerulonephritis through activation of podocyte retinoic acid receptor α.Kidney Int. 2017 Dec;92(6):1444-1457. doi: 10.1016/j.kint.2017.04.026. Epub 2017 Jul 27. Kidney Int. 2017. PMID: 28756872 Free PMC article.
-
The beneficial role of retinoids in glomerular disease.Front Med (Lausanne). 2015 Mar 23;2:16. doi: 10.3389/fmed.2015.00016. eCollection 2015. Front Med (Lausanne). 2015. PMID: 25853135 Free PMC article. Review.
-
Mechanisms of all-trans retinoic acid-induced differentiation of acute promyelocytic leukemia cells.J Biosci. 2000 Sep;25(3):275-84. doi: 10.1007/BF02703936. J Biosci. 2000. PMID: 11022230 Review.
Cited by
-
RARRES1 inhibits hepatocellular carcinoma progression and increases its sensitivity to lenvatinib through interaction with SPINK2.Biol Direct. 2024 Feb 23;19(1):15. doi: 10.1186/s13062-024-00459-0. Biol Direct. 2024. PMID: 38388961 Free PMC article.
-
Activation of the RARα Attenuated CSF Hypersecretion to Inhibit Hydrocephalus Development via Regulating the MAFB/MSR1 Pathway.Int J Mol Sci. 2023 Jan 30;24(3):2586. doi: 10.3390/ijms24032586. Int J Mol Sci. 2023. PMID: 36768908 Free PMC article.
-
Unveiling FOS as a Potential Diagnostic Biomarker and Emetine as a Prospective Therapeutic Agent for Diabetic Nephropathy.J Inflamm Res. 2023 Dec 13;16:6139-6153. doi: 10.2147/JIR.S435596. eCollection 2023. J Inflamm Res. 2023. PMID: 38107383 Free PMC article.
-
Low intake of β carotene and dietary fiber from vegetables and fruits in patients with chronic kidney disease.Sci Rep. 2022 Nov 19;12(1):19953. doi: 10.1038/s41598-022-24471-4. Sci Rep. 2022. PMID: 36402819 Free PMC article.
-
Diagnostic Yield and Benefits of Whole Exome Sequencing in CAKUT Patients Diagnosed in the First Thousand Days of Life.Kidney Int Rep. 2023 Aug 14;8(11):2439-2457. doi: 10.1016/j.ekir.2023.08.008. eCollection 2023 Nov. Kidney Int Rep. 2023. PMID: 38025229 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

