Gut microbiota shape the inflammatory response in mice with an epithelial defect
- PMID: 33645438
- PMCID: PMC7928202
- DOI: 10.1080/19490976.2021.1887720
Gut microbiota shape the inflammatory response in mice with an epithelial defect
Abstract
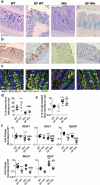
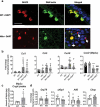
Intestinal epithelial cell endoplasmic reticulum (ER) stress has been implicated in intestinal inflammation. It remains unclear whether ER stress is an initiator of or a response to inflammation. Winnie mice, carrying a Muc2 gene mutation resulting in intestinal goblet cell ER stress, develop spontaneous colitis with a depleted mucus barrier and increased bacterial translocation. This study aims to determine whether the microbiota was required for the development of Winnie colitis, and whether protein misfolding itself can initiate inflammation directly in absence of the microbiota. To assess the role of microbiota in driving Winnie colitis, WT and Winnie mice on the same background were rederived into the germ-free facility and housed in the Trexler-type soft-sided isolators. The colitis phenotype of these mice was assessed and compared to WT and Winnie mice housed within a specific pathogen-free facility. We found that Winnie colitis was substantially reduced but not abolished under germ-free conditions. Expression of inflammatory cytokine genes was reduced but several chemokines remained elevated in absence of microbiota. Concomitantly, ER stress was also diminished, although mucin misfolding persisted. RNA-Seq revealed that Winnie differentiated colon organoids have decreased expression of the negative regulators of the inflammatory response compared to WT. This data along with the increase in Mip2a chemokine expression, suggests that the epithelial cells in the Winnie mice are more responsive to stimuli. Moreover, the data demonstrate that intestinal epithelial intrinsic protein misfolding can prime an inflammatory response without initiating the unfolded protein response in the absence of the microbiota. However, the microbiota is necessary for the amplification of colitis in Winnie mice. Genetic predisposition to mucin misfolding in secretory cells initiates mild inflammatory signals. However, the inflammatory signal sets a forward-feeding cycle establishing progressive inflammation in the presence of microbiota.Abbreviations: Endoplasmic Reticulum: ER; Mucin-2: Muc-2; GF: Germ-Free; Inflammatory Bowel Disease: IBD.
Keywords: Germ free; Inflammation; Microbiota; epithelial cells; mucin.
Figures




References
-
- Van Der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi:10.1053/j.gastro.2006.04.020. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
