Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration
- PMID: 33645550
- PMCID: PMC7919716
- DOI: 10.1172/JCI141694
Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration
Abstract
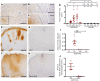
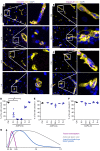
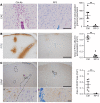
Approximately 80% of neuromyelitis optica spectrum disorder (NMOSD) patients harbor serum anti-aquaporin-4 autoantibodies targeting astrocytes in the CNS. Crucial for NMOSD lesion initiation is disruption of the blood-brain barrier (BBB), which allows the entrance of Abs and serum complement into the CNS and which is a target for new NMOSD therapies. Astrocytes have important functions in BBB maintenance; however, the influence of their loss and the role of immune cell infiltration on BBB permeability in NMOSD have not yet been investigated. Using an experimental model of targeted NMOSD lesions in rats, we demonstrate that astrocyte destruction coincides with a transient disruption of the BBB and a selective loss of occludin from tight junctions. It is noteworthy that BBB integrity is reestablished before astrocytes repopulate. Rather than persistent astrocyte loss, polymorphonuclear leukocytes (PMNs) are the main mediators of BBB disruption, and their depletion preserves BBB integrity and prevents astrocyte loss. Inhibition of PMN chemoattraction, activation, and proteolytic function reduces lesion size. In summary, our data support a crucial role for PMNs in BBB disruption and NMOSD lesion development, rendering their recruitment and activation promising therapeutic targets.
Keywords: Demyelinating disorders; Neuroscience; Neutrophils; Tight junctions.
Conflict of interest statement
Figures



Similar articles
-
Staging of astrocytopathy and complement activation in neuromyelitis optica spectrum disorders.Brain. 2021 Sep 4;144(8):2401-2415. doi: 10.1093/brain/awab102. Brain. 2021. PMID: 33711152
-
Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment.J Immunol. 2008 Oct 15;181(8):5730-7. doi: 10.4049/jimmunol.181.8.5730. J Immunol. 2008. PMID: 18832732
-
Purified IgG from aquaporin-4 neuromyelitis optica spectrum disorder patients alters blood-brain barrier permeability.PLoS One. 2020 Sep 3;15(9):e0238301. doi: 10.1371/journal.pone.0238301. eCollection 2020. PLoS One. 2020. PMID: 32881954 Free PMC article.
-
Blood-Brain Barrier Disruption in Neuroimmunological Disease.Int J Mol Sci. 2024 Oct 2;25(19):10625. doi: 10.3390/ijms251910625. Int J Mol Sci. 2024. PMID: 39408955 Free PMC article. Review.
-
[Clinical concept, etiology and pathology of neuromyelitis optica].Nihon Rinsho. 2014 Nov;72(11):1897-902. Nihon Rinsho. 2014. PMID: 25518368 Review. Japanese.
Cited by
-
Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier.Cells. 2024 Jan 12;13(2):150. doi: 10.3390/cells13020150. Cells. 2024. PMID: 38247841 Free PMC article. Review.
-
Stage-dependent immunity orchestrates AQP4 antibody-guided NMOSD pathology: a role for netting neutrophils with resident memory T cells in situ.Acta Neuropathol. 2024 Apr 24;147(1):76. doi: 10.1007/s00401-024-02725-x. Acta Neuropathol. 2024. PMID: 38658413
-
Possible Roles of Extracellular Vesicles in the Pathogenesis and Interventions of Immune-Mediated Central Demyelinating Diseases.Exp Neurobiol. 2024 Apr 30;33(2):47-67. doi: 10.5607/en24002. Exp Neurobiol. 2024. PMID: 38724476 Free PMC article. Review.
-
Long-term Effects of IL-6 Receptor Blockade Therapy on Regulatory Lymphocytes and Neutrophils in Neuromyelitis Optica Spectrum Disorder.Neurol Neuroimmunol Neuroinflamm. 2023 Oct 20;11(1):e200173. doi: 10.1212/NXI.0000000000200173. Print 2024 Jan. Neurol Neuroimmunol Neuroinflamm. 2023. PMID: 37863660 Free PMC article.
-
An easy-to-perform method for microvessel isolation and primary brain endothelial cell culture to study Alzheimer's disease.Heliyon. 2024 Jun 15;10(12):e33077. doi: 10.1016/j.heliyon.2024.e33077. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38994107 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

