Functional Assessment of Intestinal Permeability and Neutrophil Transepithelial Migration in Mice using a Standardized Intestinal Loop Model
- PMID: 33645571
- PMCID: PMC11404721
- DOI: 10.3791/62093
Functional Assessment of Intestinal Permeability and Neutrophil Transepithelial Migration in Mice using a Standardized Intestinal Loop Model
Abstract
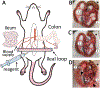
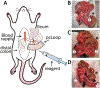
The intestinal mucosa is lined by a single layer of epithelial cells that forms a dynamic barrier allowing paracellular transport of nutrients and water while preventing passage of luminal bacteria and exogenous substances. A breach of this layer results in increased permeability to luminal contents and recruitment of immune cells, both of which are hallmarks of pathologic states in the gut including inflammatory bowel disease (IBD). Mechanisms regulating epithelial barrier function and transepithelial migration (TEpM) of polymorphonuclear neutrophils (PMN) are incompletely understood due to the lack of experimental in vivo methods allowing quantitative analyses. Here, we describe a robust murine experimental model that employs an exteriorized intestinal segment of either ileum or proximal colon. The exteriorized intestinal loop (iLoop) is fully vascularized and offers physiological advantages over ex vivo chamber-based approaches commonly used to study permeability and PMN migration across epithelial cell monolayers. We demonstrate two applications of this model in detail: (1) quantitative measurement of intestinal permeability through detection of fluorescence-labeled dextrans in serum after intraluminal injection, (2) quantitative assessment of migrated PMN across the intestinal epithelium into the gut lumen after intraluminal introduction of chemoattractants. We demonstrate feasibility of this model and provide results utilizing the iLoop in mice lacking the epithelial tight junction-associated protein JAM-A compared to controls. JAM-A has been shown to regulate epithelial barrier function as well as PMN TEpM during inflammatory responses. Our results using the iLoop confirm previous studies and highlight the importance of JAM-A in regulation of intestinal permeability and PMN TEpM in vivo during homeostasis and disease. The iLoop model provides a highly standardized method for reproducible in vivo studies of intestinal homeostasis and inflammation and will significantly enhance understanding of intestinal barrier function and mucosal inflammation in diseases such as IBD.
Figures
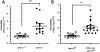

References
-
- Jump RL, Levine AD Mechanisms of natural tolerance in the intestine: implications for inflammatory bowel disease. Inflammatory Bowel Diseases. 10 (4), 462–478 (2004). - PubMed
-
- Peeters M. et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 113 (3), 802–807 (1997). - PubMed
-
- Chin AC, Parkos CA Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Annals of the New York Academy of Sciences. 1072, 276–287 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
