A Murine Tail Lymphedema Model
- PMID: 33645579
- PMCID: PMC8056335
- DOI: 10.3791/61848
A Murine Tail Lymphedema Model
Abstract
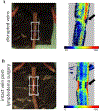
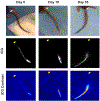
Lymphedema is extremity swelling caused by lymphatic dysfunction. The affected limb enlarges because of accumulation of fluid, adipose, and fibrosis. There is no cure for this disease. A mouse tail model that uses a focal full thickness skin excision near the base of the tail, resulting in tail swelling, has been used to study lymphedema. However, this model may result in vascular comprise and consequent tail necrosis and early tail swelling resolution, limiting its clinical translatability. The chronic murine tail lymphedema model induces sustained lymphedema over 15 weeks and a reliable perfusion to the tail. Enhancements of the traditional murine tail lymphedema model include 1) precise full thickness excision and lymphatic clipping using a surgical microscope, 2) confirmation of post-operative arterial and venous perfusion using high resolution laser speckle, and 3) functional assessment using indocyanine green near infrared laser lymphangiography. We also use tissue nanotransfection technology (TNT) for novel non-viral, transcutaneous, focal delivery of genetic cargo to the mouse tail vasculature.
Conflict of interest statement
Disclosures
The authors have no competing conflicts of interest.
Figures



References
-
- Mendoza N, Li A, Gill A, Tyring S Filariasis: diagnosis and treatment. Dermatology and Therapy. 22 (6), 475–490 (2009). - PubMed
-
- Rockson SG, Rivera KK Estimating the population burden of lymphedema. Annals of the New York Academy of Sciences. 1131, 147–154 (2008). - PubMed
-
- Soran A et al. Breast cancer-related lymphedema--what are the significant predictors and how they affect the severity of lymphedema? Breast Journal. 12 (6), 536–543 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
