Molecular mechanisms of An-Chuan Granule for the treatment of asthma based on a network pharmacology approach and experimental validation
- PMID: 33645621
- PMCID: PMC7990088
- DOI: 10.1042/BSR20204247
Molecular mechanisms of An-Chuan Granule for the treatment of asthma based on a network pharmacology approach and experimental validation
Abstract
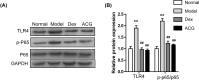
An-Chuan Granule (ACG), a traditional Chinese medicine (TCM) formula, is an effective treatment for asthma but its pharmacological mechanism remains poorly understood. In the present study, network pharmacology was applied to explore the potential mechanism of ACG in the treatment of asthma. The tumor necrosis factor (TNF), Toll-like receptor (TLR), and Th17 cell differentiation-related, nucleotide-binding oligomerization domain (NOD)-like receptor, and NF-kappaB pathways were identified as the most significant signaling pathways involved in the therapeutic effect of ACG on asthma. A mouse asthma model was established using ovalbumin (OVA) to verify the effect of ACG and the underlying mechanism. The results showed that ACG treatment not only attenuated the clinical symptoms, but also reduced inflammatory cell infiltration, mucus secretion and MUC5AC production in lung tissue of asthmatic mice. In addition, ACG treatment notably decreased the inflammatory cell numbers in bronchoalveolar lavage fluid (BALF) and the levels of pro-inflammatory cytokines (including IL-6, IL-17, IL-23, TNF-alpha, IL-1beta and TGF-beta) in lung tissue of asthmatic mice. In addition, ACG treatment remarkably down-regulated the expression of TLR4, p-P65, NLRP3, Caspase-1 and adenosquamous carcinoma (ASC) in lung tissue. Further, ACG treatment decreased the expression of receptor-related orphan receptor (RORγt) in lung tissue but increased that of Forkhead box (Foxp3). In conclusion, the above results demonstrate that ACG alleviates the severity of asthma in a ´multi-compound and multi-target' manner, which provides a basis for better understanding of the application of ACG in the treatment of asthma.
Keywords: An-Chuan Granule; Asthma; Inflammation; Network pharmacology; Th17/Treg balance.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interests associated with the manuscript.
Figures





Similar articles
-
Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model.J Ethnopharmacol. 2015 Apr 22;164:368-77. doi: 10.1016/j.jep.2015.01.016. Epub 2015 Jan 24. J Ethnopharmacol. 2015. PMID: 25625352
-
Epigallocatechin gallate ameliorates airway inflammation by regulating Treg/Th17 imbalance in an asthmatic mouse model.Int Immunopharmacol. 2019 Jul;72:422-428. doi: 10.1016/j.intimp.2019.04.044. Epub 2019 Apr 28. Int Immunopharmacol. 2019. PMID: 31030098
-
[Effects of vasoactive intestinal peptide on airway inflammation and Th17/Treg balance in asthmatic mice].Zhongguo Dang Dai Er Ke Za Zhi. 2017 Jun;19(6):699-704. doi: 10.7499/j.issn.1008-8830.2017.06.017. Zhongguo Dang Dai Er Ke Za Zhi. 2017. PMID: 28606240 Free PMC article. Chinese.
-
The Role of Th17/Treg Axis in the Traditional Chinese Medicine Intervention on Immune-Mediated Inflammatory Diseases: A Systematic Review.Am J Chin Med. 2020;48(3):535-558. doi: 10.1142/S0192415X20500275. Epub 2020 Apr 29. Am J Chin Med. 2020. PMID: 32345031
-
Herbal medicine for the treatment of obesity-associated asthma: a comprehensive review.Front Pharmacol. 2023 May 11;14:1186060. doi: 10.3389/fphar.2023.1186060. eCollection 2023. Front Pharmacol. 2023. PMID: 37251328 Free PMC article. Review.
Cited by
-
MDSCs Aggravate the Asthmatic Progression in Children and OVA-Allergic Mice by Regulating the Th1/Th2/Th17 Responses.Evid Based Complement Alternat Med. 2022 Aug 22;2022:6157385. doi: 10.1155/2022/6157385. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36045657 Free PMC article.
-
NOTCH pathway was involved in Kaempferol 3-O-gentiobioside attenuated airway inflammation and mucus hypersecretion.Sci Rep. 2025 Mar 26;15(1):10383. doi: 10.1038/s41598-025-95280-8. Sci Rep. 2025. PMID: 40140655 Free PMC article.
-
Obesity Enhances Non-Th2 Airway Inflammation in a Murine Model of Allergic Asthma.Int J Mol Sci. 2024 Jun 4;25(11):6170. doi: 10.3390/ijms25116170. Int J Mol Sci. 2024. PMID: 38892358 Free PMC article.
-
Research hotspot and frontier analysis of traditional Chinese medicine in asthma using bibliometric methods from 1991 to 2021.J Allergy Clin Immunol Glob. 2022 Sep 2;1(4):185-197. doi: 10.1016/j.jacig.2022.07.004. eCollection 2022 Nov. J Allergy Clin Immunol Glob. 2022. PMID: 37779535 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

