Nonmotor symptom burden grading as predictor of cognitive impairment in Parkinson's disease
- PMID: 33645912
- PMCID: PMC8119808
- DOI: 10.1002/brb3.2086
Nonmotor symptom burden grading as predictor of cognitive impairment in Parkinson's disease
Abstract
Background: Identifying predictors of incident cognitive impairment (CI), one of the most problematic long-term outcomes, in Parkinson's disease (PD) is highly relevant for personalized medicine and prognostic counseling. The Nonmotor Symptoms Scale (NMSS) provides a global clinical assessment of a range of NMS, reflecting NMS burden (NMSB), and thus may assist in the identification of an "at-risk" CI group based on overall NMSB cutoff scores.
Methods: To investigate whether specific patterns of PD NMS profiles predict incident CI, we performed a retrospective longitudinal study on a convenience sample of 541 nondemented PD patients taking part in the Nonmotor Longitudinal International Study (NILS) cohort, with Mini-Mental State Examination (MMSE), NMSS, and Scales for Outcomes in PD Motor Scale (SCOPA Motor) scores at baseline and last follow-up (mean 3.2 years) being available.
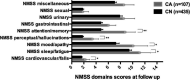
Results: PD patients with incident CI (i.e., MMSE score ≤ 25) at last follow-up (n = 107) had severe overall NMSB level, significantly worse NMSS hallucinations/perceptual problems and higher NMSS attention/memory scores at baseline. Patients with CI also were older and with more advanced disease, but with no differences in disease duration, dopamine replacement therapy, sex, and comorbid depression, anxiety, and sleep disorders.
Conclusions: Our findings suggest that a comprehensive baseline measure of NMS and in particular hallucinations and perceptual problems assessed with a validated single instrument can be used to predict incident CI in PD. This approach provides a simple, holistic strategy to predict future CI in this population.
Keywords: MMSE; Nonmotor symptom burden grading; Nonmotor symptoms; Parkinson's disease; cognitive impairment.
© 2021 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Oikonomou has been supported by the European Academy of Neurology Clinical Fellowship Programme 2019. Dr. van Wamelen reports grants and personal fees from Britannia Pharmaceuticals, personal fees from Invisio Pharmaceuticals, and personal fees from Abbvie. Dr. Weintraub has received research funding or support from Michael J. Fox Foundation for Parkinson's Research, Alzheimer's Therapeutic. Dr. Martinez‐Martin Research Initiative (ATRI), Alzheimer's Disease Cooperative Study (ADCS), the International Parkinson and Movement Disorder Society (IPMDS), and National Institute on Aging (NIA); honoraria for consultancy from Acadia, Aptinyx, Biogen, CHDI Foundation, Clintrex LLC, Eisai, Enterin, F. Hoffmann‐La Roche Ltd, Ferring, Janssen, Otsuka, Promentis, Sage, Signant Health, Sunovion, and Takeda; and license fee payments from the University of Pennsylvania for the QUIP and QUIP‐RS. Dr. Martinez‐Martin has received honoraria from National School of Public Health (ISCIII), Britannia, and Editorial Viguera for lecturing in courses, and from Bial, and Zambon for advice in clinical‐epidemiological studies. From the International Parkinson and Movement Disorder Society has received honoraria for management of the Program on Rating Scales, travel grant for attending the International Congress 2019, and financial support for development and validation of the MDS‐NMS. Dr Ffytche, Dr. Aarsland and Dr. Rodriguez‐Blazquez have nothing to disclose. Dr. Leta reports grants from Parkinson's UK, grants from Bial UK Ltd, other from Britannia pharmaceuticals, and other from Invisio Pharmaceuticals. Ms. Borley, Ms. Sportelli, Dr. Trivedi, Ms. Podlewska, Dr. Rukavina, and Dr. Lazcano‐Ocampo have nothing to disclose. Mrs. Rizos has received salary support from the National Institute of Health Research (NIHR) Clinical Research Network (CRN) South London and speaker honorarium from Britannia Pharmaceuticals Ltd. Dr. Ray Chaudhuri has received honoraria for advisory boards: AbbVie, Britannia Pharmaceuticals, UCB, Pfizer, Jazz Pharma, GKC, Bial, Cynapsus, Novartis, Lobsor, Stada, Medtronic, Zambon, Profile Pharma, Sunovion, Roche, Theravance Biopharma, Scion; honoraria for lectures from AbbVie, Britannia Pharmaceuticals, UCB, Mundipharma, Zambon, Novartis, Boeringer Ingelheim Neuroderm, Sunovion; grants (Investigator Initiated) from Britannia Pharmaceuticals, AbbVie, UCB, GKC, Bial, and academic grants from EU (Horizon 2020), IMI EU, Parkinson's UK, NIHR, PDNMG, Kirby Laing Foundation, NPF, MRC.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

