A Thiol-Mediated Three-Step Ring Expansion Cascade for the Conversion of Indoles into Functionalized Quinolines
- PMID: 33645997
- PMCID: PMC8041380
- DOI: 10.1021/acs.orglett.1c00205
A Thiol-Mediated Three-Step Ring Expansion Cascade for the Conversion of Indoles into Functionalized Quinolines
Abstract
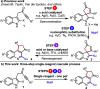
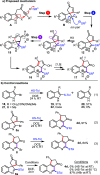
An operationally simple, high yielding three-step cascade process is described for the direct conversion of indole-tethered ynones into functionalized quinolines. A single "multitasking" thiol reagent is used to promote a three-step dearomatizing spirocyclization, nucleophilic substitution, and one-atom ring expansion reaction cascade under remarkably mild conditions. In addition, a novel route to thio-oxindoles is described, which was discovered by serendipity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
-
For reviews of tandem/cascade reactions, see:
- Taylor R. J. K.; Reid M.; Foot J.; Raw S. A. Tandem Oxidation Processes using Manganese Dioxide: Discovery, Applications and Current Studies. Acc. Chem. Res. 2005, 38, 851–869. 10.1021/ar050113t. - DOI - PubMed
- Nicolaou K. C.; Chen J. S. The art of total synthesis through cascade reactions. Chem. Soc. Rev. 2009, 38, 2993–3009. 10.1039/b903290h. - DOI - PMC - PubMed
- Plesniak M. P.; Huang H.-M.; Procter D. J. Radical cascade reactions triggered by single electron transfer. Nat. Rev. Chem. 2017, 1, 0077. 10.1038/s41570-017-0077. - DOI
- Prabagar B.; Ghosh N.; Sahoo A. K. Cyclization and Cycloisomerization of π-Tethered Ynamides: An Expedient Synthetic Method to Construct Carbo- and Heterocycles. Synlett 2017, 28, 2539–2555. 10.1055/s-0036-1590877. - DOI
- Sperl J. M.; Sieber V. Multienzyme Cascade Reactions–Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. 10.1021/acscatal.7b03440. - DOI
- Huang H.-M.; Garduño-Castro M. H.; Morrill C.; Procter D. J. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 2019, 48, 4626–4638. 10.1039/C8CS00947C. - DOI - PubMed
-
-
-
For selected examples from our own groups, see:
- Raw S.; Taylor R. J. K. Cascade Reactions of Substituted 1,2,4-Triazines: Rapid Access to Nitrogen-Containing Polycycles. J. Am. Chem. Soc. 2004, 126, 12260–12261. 10.1021/ja045780g. - DOI - PubMed
- Edwards M. G.; Kenworthy M.; Kitson R. A. A.; Scott M.; Taylor R. J. K. The Telescoped Intramolecular Michael/Olefination (TIMO) Approach to α-Alkylidene γ-Butyrolactones: Synthesis of (+)-Paeonilactone B. Angew. Chem., Int. Ed. 2008, 47, 1935–1937. 10.1002/anie.200705329. - DOI - PubMed
- Lawer A.; Rossi-Ashton J. A.; Stephens T. C.; Challis B. J.; Epton R. G.; Lynam J. M.; Unsworth W. P. Internal Nucleophilic Catalyst Mediated Cyclisation/Ring Expansion Cascades for the Synthesis of Medium-Sized Lactones and Lactams. Angew. Chem., Int. Ed. 2019, 58, 13942–13947. 10.1002/anie.201907206. - DOI - PubMed
-
-
-
For studies relating to step 1, see:
- Ekebergh A.; Börje A.; Mårtensson J. Total Synthesis of Nostodione A, a Cyanobacterial Metabolite. Org. Lett. 2012, 14, 6274–6277. 10.1021/ol303036j. - DOI - PubMed
- James M. J.; Cuthbertson J. D.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Silver(I)- or Copper(II)-Mediated Dearomatization of Aromatic Ynones: Direct Access to Spirocyclic Scaffolds. Angew. Chem., Int. Ed. 2015, 54, 7640–7643. 10.1002/anie.201501812. - DOI - PubMed
- Clarke A. K.; James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Silica-Supported Silver Nitrate as a Highly Active Dearomatizing Spirocyclization Catalyst: Synergistic Alkyne Activation by Silver Nanoparticles and Silica. Angew. Chem., Int. Ed. 2016, 55, 13798–13802. 10.1002/anie.201608263. - DOI - PubMed
- Liddon J. T. R.; Rossi-Ashton J. A.; Clarke A. K.; Lynam J. M.; Taylor R. J. K.; Unsworth W. P. Divergent reactivity of indole tethered ynones with silver(I) and gold(I) catalysis: a combined synthetic and computational study. Synthesis 2018, 50, 4829–4836. 10.1055/s-0037-1610181. - DOI
- Fedoseev P.; Coppola G.; Ojeda G. M.; Van der Eycken E. V. Synthesis of Spiroindolenines by Intramolecular Ipso-Iodocyclization of Indol Ynones. Chem. Commun. 2018, 54, 3625–3628. 10.1039/C8CC01474D. - DOI - PubMed
- Han G.; Xue L.; Zhao L.; Zhu T.; Hou J.; Song Y.; Liu Y. Access to CF3-Containing Cyclopentaquinolinone Derivatives from Indolyl-ynones via Silver-Catalyzed One-pot Reaction. Adv. Synth. Catal. 2019, 361, 678–682. 10.1002/adsc.201801482. - DOI
- Rossi-Ashton J. A.; Clarke A. K.; Taylor R. J. K.; Unsworth W. P. Modular Synthesis of Polycyclic Alkaloid Scaffolds via an Enantioselective Dearomative Cascade. Org. Lett. 2020, 22, 1175–1181. 10.1021/acs.orglett.0c00053. - DOI - PMC - PubMed
-
-
-
For radical dearomatizing spirocyclization reactions of indoles of the form 1, see:
- Ho H. E.; Pagano A.; Rossi-Ashton J. A.; Donald J. R.; Epton R. G.; Churchill J. C.; James M. J.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Visible-light-induced intramolecular charge transfer in the radical spirocyclisation of indole-tethered ynones. Chem. Sci. 2020, 11, 1353–1360. 10.1039/C9SC05311E. - DOI - PMC - PubMed
- Chengwen Li C.; Xue L.; Zhou J.; Zhao Y.; Han G.; Hou J.; Song Y.; Liu Y. Copper-Catalyzed Trifluoromethylation of Ynones Coupled with Dearomatizing Spirocyclization of Indoles: Access to CF3-Containing Spiro[cyclopentane-1,3′-indole]. Org. Lett. 2020, 22, 3291–3296. 10.1021/acs.orglett.0c01097. - DOI - PubMed
- Zhou X.-Ji.; Liu H.-Y.; Mo Z.-Y.; Ma X.-L.; Chen Y.-Y.; Tang H.-T.; Pan Y.-M.; Xu Y.-L. Visible-Light-Promoted Selenylative Spirocyclization of Indolyl-ynones toward the Formation of 3-Selenospiroindolenine Anticancer Agents. Chem. - Asian J. 2020, 15, 1536–1539. 10.1002/asia.202000298. - DOI - PubMed
-
-
- For studies relating to step 2, see:Liddon J. T. R.; Clarke A. K.; Taylor R. J. K.; Unsworth W. P. Preparation and Reactions of Indoleninyl Halides: Scaffolds for the Synthesis of Spirocyclic Indole Derivatives. Org. Lett. 2016, 18, 6328–6331. 10.1021/acs.orglett.6b03221. - DOI - PubMed
-
and ref (4b).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

