Comparison of methods used for evaluation of mutagenicity/genotoxicity of model chemicals - parabens
- PMID: 33646007
- PMCID: PMC8603696
- DOI: 10.33549/physiolres.934615
Comparison of methods used for evaluation of mutagenicity/genotoxicity of model chemicals - parabens
Abstract
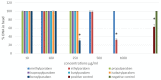
Growing worldwide efforts to replace (reduce) animal testing and to improve alternative in vitro tests which may be more efficient in terms of both time, cost and scientific validity include also genotoxicity/mutagenicity endpoints. The aim of the review article was to summarize currently available in vitro testing approaches in this field, their regulatory acceptance and recommended combinations for classification of chemicals. A study using the combination of Comet Assay performed on two cell lines and the Chromosomal Aberration test on human peripheral lymphocytes was performed with the aim to predict the genotoxic potential of selected paraben esters, serving as a model chemical group. Parabens are widely used in consumer products as preservatives and have been reported to exhibit inconclusive results in numerous genotoxicity studies. The Comet Assay identified Ethylparaben and Benzylparaben as potentially genotoxic. The Chromosomal Aberration test revealed weak genotoxic potential in case of Ethylparaben and positive genotoxicity in case of Butylparaben, Propylparaben and Isopropylparaben. The main reasons for variability seem to be limited water solubility of parabens, determining their bioavailability at the cellular level, and absence of metabolic activation in the Comet Assay. The results confirmed that the Comet Assay should serve as a screening test and should not be used as a stand-alone method for classification of genotoxicity. The weight of evidence approach in risk assessment should be supported with data generated with the use of human relevant in vitro methods based on cells / tissues of human origin.
Conflict of interest statement
There is no conflict of interest.
Figures



References
-
- ALÉPÉE N, BAHINSKI A, DANESHIAN M, De WEVER B, FRITSCHE E, GOLDBERG A, HANSMANN J, HARTUNG T, HAYCOCK J, HOGBERG H, HOELTING L, KELM JM, KADEREIT S, McVEY E, LANDSIEDEL R, LEIST M, LÜBBERSTEDT M, NOOR F, PELLEVOISIN C, PETERSOHN D, PFANNENBECKER U, REISINGER K, RAMIREZ T, ROTHEN-RUTISHAUSER B, SCHÄFER-KORTING M, ZEILINGER K, ZURICH MG. t4 workshop report: State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. ALTEX. 2014;31:441–477. doi: 10.14573/altex1406111. - DOI - PMC - PubMed
-
- ANDERSEN ME, BETTS K, DRAGAN Y, FITZPATRICK S, GOODMAN JL, HARTUNG T, HIMMELFARB J, INGBER DE, JACOBS A, KAVLOCK R, KOLAJA K, STEVENS JL, TAGLE D, LANSING TAYLOR D, THROCKMORTON D. Developing microphysiological systems for use as regulatory tools - challenges and opportunities. ALTEX. 2014;31:64–367. doi: 10.14573/altex.1405151. - DOI - PMC - PubMed
