Oral Dysbiosis and Inflammation in Parkinson's Disease
- PMID: 33646178
- PMCID: PMC8150470
- DOI: 10.3233/JPD-202459
Oral Dysbiosis and Inflammation in Parkinson's Disease
Abstract
Background: Oral microbiota has largely escaped attention in Parkinson's disease (PD), despite its pivotal role in maintaining oral and systemic health.
Objective: The aim of our study was to examine the composition of the oral microbiota and the degree of oral inflammation in PD.
Methods: Twenty PD patients were compared to 20 healthy controls. Neurological, periodontal and dental examinations were performed as well as dental scaling and gingival crevicular fluid sampling for cytokines measurement (interleukine (IL)-1β, IL-6, IL-1 receptor antagonist (RA), interferon-γ and tumor necrosis factor (TNF)-α). Two months later, oral microbiota was sampled from saliva and subgingival dental plaque. A 16S rRNA gene amplicon sequencing was used to assess bacterial communities.
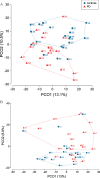
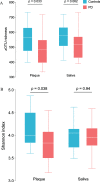
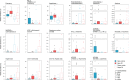
Results: PD patients were in the early and mid-stage phases of their disease (Hoehn & Yahr 2-2.5). Dental and periodontal parameters did not differ between groups. The levels of IL-1β and IL-1RA were significantly increased in patients compared to controls with a trend for an increased level of TNF-α in patients. Both saliva and subgingival dental plaque microbiota differed between patients and controls. Streptococcus mutans, Kingella oralis, Actinomyces AFQC_s, Veillonella AFUJ_s, Scardovia, Lactobacillaceae, Negativicutes and Firmicutes were more abundant in patients, whereas Treponema KE332528_s, Lachnospiraceae AM420052_s, and phylum SR1 were less abundant.
Conclusion: Our findings show that the oral microbiome is altered in early and mid-stage PD. Although PD patients had good dental and periodontal status, local inflammation was already present in the oral cavity. The relationship between oral dysbiosis, inflammation and the pathogenesis of PD requires further study.
Keywords: Oral microbiome; Parkinson’s disease; biomarker; cytokine; inflammation; microbiota; non-motor symptoms.
Conflict of interest statement
The authors have no financial disclosures and no conflicts of interest concerning the research related to the manuscript.
Figures
References
-
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3, 17013. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388, 839–840. - PubMed
-
- Mu L, Chen J, Sobotka S, Nyirenda T, Benson B, Gupta F, Sanders I, Adler CH, Caviness JN, Shill HA, Sabbagh M, Samanta JE, Sue LI, Beach TG, Arizona Parkinson’s Disease Consortium (2015) Alpha-synuclein pathology in sensory nerve terminals of the upper aerodigestive tract of Parkinson’s disease patients. Dysphagia 30, 404–417. - PMC - PubMed
-
- Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P (2016) Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 79, 940–949. - PubMed
-
- Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, Marti C, Serradell M, Lomena F, Alos L, Gaig C, Santamaria J, Gelpi E (2016) Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. Lancet Neurol 15, 708–718. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

