Immune System Investigation Using Parasitic Helminths
- PMID: 33646858
- PMCID: PMC8162934
- DOI: 10.1146/annurev-immunol-093019-122827
Immune System Investigation Using Parasitic Helminths
Abstract
Coevolutionary adaptation between humans and helminths has developed a finely tuned balance between host immunity and chronic parasitism due to immunoregulation. Given that these reciprocal forces drive selection, experimental models of helminth infection are ideally suited for discovering how host protective immune responses adapt to the unique tissue niches inhabited by these large metazoan parasites. This review highlights the key discoveries in the immunology of helminth infection made over the last decade, from innate lymphoid cells to the emerging importance of neuroimmune connections. A particular emphasis is placed on the emerging areas within helminth immunology where the most growth is possible, including the advent of genetic manipulation of parasites to study immunology and the use of engineered T cells for therapeutic options. Lastly,we cover the status of human challenge trials with helminths as treatment for autoimmune disease, which taken together, stand to keep the study of parasitic worms at the forefront of immunology for years to come.
Keywords: animal models; helminth; innate immunity; lymphocytes; neuroimmunology; transgenesis.
Figures
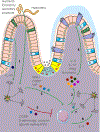

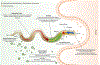

References
-
- Sianto L, Chame M, Silva CS, Goncalves ML, Reinhard K, Fugassa M, Araujo A. 2009. Animal helminths in human archaeological remains: a review of zoonoses in the past. Rev. Inst. Med. Trop. Sao Paulo 51:119–30 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

