Maltohexaose-indocyanine green (MH-ICG) for near infrared imaging of endocarditis
- PMID: 33647027
- PMCID: PMC7920357
- DOI: 10.1371/journal.pone.0247673
Maltohexaose-indocyanine green (MH-ICG) for near infrared imaging of endocarditis
Abstract
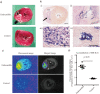
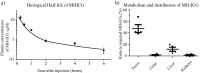
Infectious endocarditis is a life-threatening disease, and diagnostics are urgently needed to accurately diagnose this disease especially in the case of prosthetic valve endocarditis. We show here that maltohexaose conjugated to indocyanine green (MH-ICG) can detect Staphylococcus aureus (S. aureus) infection in a rat model of infective endocarditis. The affinity of MH-ICG to S. aureus was determined and had a Km and Vmax of 5.4 μM and 3.0 X 10-6 μmol/minutes/108 CFU, respectively. MH-ICG had no detectable toxicity to mammalian cells at concentrations as high as 100 μM. The in vivo efficiency of MH-ICG in rats was evaluated using a right heart endocarditis model, and the accumulation of MH-ICG in the bacterial vegetations was 2.5 ± 0.2 times higher than that in the control left ventricular wall. The biological half-life of MH-ICG in healthy rats was 14.0 ± 1.3 minutes, and approximately 50% of injected MH-ICG was excreted into the feces after 24 hours. These data demonstrate that MH-ICG was internalized by bacteria with high specificity and that MH-ICG specifically accumulated in bacterial vegetations in a rat model of endocarditis. These results demonstrate the potential efficacy of this agent in the detection of infective endocarditis.
Conflict of interest statement
Kiyoko Takemiya, W. Robert Taylor, Niren Murthy, and Mark M. Goodman have equity interest in Microbial Medical, Inc which is related to this study. Joachim J. Røise, Maomao He, Chung Taing, and Alexander G. Rodriguez have no conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

