Notum deacylates octanoylated ghrelin
- PMID: 33647468
- PMCID: PMC8010218
- DOI: 10.1016/j.molmet.2021.101201
Notum deacylates octanoylated ghrelin
Abstract
Objectives: The only proteins known to be modified by O-linked lipidation are Wnts and ghrelin, and enzymatic removal of this post-translational modification inhibits ligand activity. Indeed, the Wnt-deacylase activity of Notum is the basis of its ability to act as a feedback inhibitor of Wnt signalling. Whether Notum also deacylates ghrelin has not been determined.
Methods: We used mass spectrometry to assay ghrelin deacylation by Notum and co-crystallisation to reveal enzyme-substrate interactions at the atomic level. CRISPR/Cas technology was used to tag endogenous Notum and assess its localisation in mice while liver-specific Notum knock-out mice allowed us to investigate the physiological role of Notum in modulating the level of ghrelin deacylation.
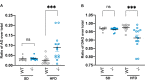
Results: Mass spectrometry detected the removal of octanoyl from ghrelin by purified active Notum but not by an inactive mutant. The 2.2 Å resolution crystal structure of the Notum-ghrelin complex showed that the octanoyl lipid was accommodated in the hydrophobic pocket of the Notum. The knock-in allele expressing HA-tagged Notum revealed that Notum was produced in the liver and present in the bloodstream, albeit at a low level. Liver-specific inactivation of Notum in animals fed a high-fat diet led to a small but significant increase in acylated ghrelin in the circulation, while no such increase was seen in wild-type animals on the same diet.
Conclusions: Overall, our data demonstrate that Notum can act as a ghrelin deacylase, and that this may be physiologically relevant under high-fat diet conditions. Our study therefore adds Notum to the list of enzymes, including butyrylcholinesterase and other carboxylesterases, that modulate the acylation state of ghrelin. The contribution of multiple enzymes could help tune the activity of this important hormone to a wide range of physiological conditions.
Keywords: Crystal structure; Deacylation; Ghrelin; Metabolism; Notum.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures



References
-
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. - PubMed
-
- Takaya K., Ariyasu H., Kanamoto N., Iwakura H., Yoshimoto A., Harada M. Ghrelin strongly stimulates growth hormone release in humans. Journal of Clinical Endocrinology & Metabolism. 2000;85(12):4908–4911. - PubMed
-
- Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. - PubMed
-
- Yang J., Brown M.S., Liang G., Grishin N.V., Goldstein J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

