Evaluation of Lumipulse® G SARS-CoV-2 antigen assay automated test for detecting SARS-CoV-2 nucleocapsid protein (NP) in nasopharyngeal swabs for community and population screening
- PMID: 33647511
- PMCID: PMC7908845
- DOI: 10.1016/j.ijid.2021.02.098
Evaluation of Lumipulse® G SARS-CoV-2 antigen assay automated test for detecting SARS-CoV-2 nucleocapsid protein (NP) in nasopharyngeal swabs for community and population screening
Abstract
Objectives: To compare the Lumipulse® SARS-CoV-2 antigen test with the gold standard real-time reverse transcription-polymerase chain reaction (RT-PCR) for diagnosis of SARS-CoV-2 infection and to evaluate its role in screening programs.
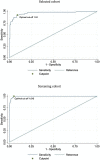
Methods: Lumipulse® SARS-CoV-2 antigen assay was compared with the gold standard RT-PCR test in a selected cohort of 226 subjects with suspected SARS-CoV-2 infection, and its accuracy was evaluated. Subsequently, the test was administered to a real-life screening cohort of 1738 cases. ROC analysis was performed to explore test features and cutoffs. All tests were performed in the regional reference laboratory in Umbria, Italy.
Results: A 42.0% positive result at RT-PCR was observed in the selected cohort. The Lumipulse® system showed 92.6% sensitivity (95% CI 85.4-97.0%) and 90.8% specificity (95% CI 84.5-95.2%) at 1.24 pg/mL optimal cutoff. In the screening cohort, characterized by 5.2% prevalence of infection, Lumipulse® assay showed 100% sensitivity (95% CI 96.0-100.0%) and 94.8% specificity (95% CI 93.6-95.8%) at 1.645 pg/mL optimal cutoff; the AUC was 97.4%, NPV was 100% (95% CI 99.8-100.0%) and PPV was 51.1% (95% CI 43.5-58.7%).
Conclusions: The Lumipulse® SARS-CoV-2 antigen assay can be safely employed in the screening strategies in small and large communities and in the general population.
Keywords: Antigen NP; COVID-19; Diagnosis; Lumipulse®; SARS-CoV-2; Screening.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

