Low doses of zeolitic imidazolate framework-8 nanoparticles alter the actin organization and contractility of vascular smooth muscle cells
- PMID: 33647611
- PMCID: PMC8144069
- DOI: 10.1016/j.jhazmat.2021.125514
Low doses of zeolitic imidazolate framework-8 nanoparticles alter the actin organization and contractility of vascular smooth muscle cells
Abstract
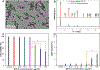
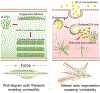
Zeolitic imidazolate framework-8 (ZIF-8) nanoparticles have emerged as a promising platform for drug delivery and controlled release. Considering most ZIF-8 nanoparticle drug carriers are designed to be administered intravenously, and thus would directly contact vascular smooth muscle cells (VSMCs) in many circumstances, the potential interactions of ZIF-8 nanoparticles with VSMCs require investigation. Here, the effects of low doses of ZIF-8 nanoparticles on VSMC morphology, actin organization, and contractility are investigated. Two nanoscale imaging tools, atomic force microscopy, and direct stochastic optical reconstruction microscopy, show that even at the concentrations (12.5 and 25 µg/ml) that were deemed "safe" by conventional biochemical cell assays (MTT and LDH assays), ZIF-8 nanoparticles can still cause changes in cell morphology and actin cytoskeleton organization at the cell apical and basal surfaces. These cytoskeletal structural changes impair the contractility function of VSMCs in response to Angiotensin II, a classic vasoconstrictor. Based on intracellular zinc and actin polymerization assays, we conclude that the increased intracellular Zn2+ concentration due to the uptake and dissociation of ZIF-8 nanoparticles could cause the actin cytoskeleton dis-organization, as the elevated Zn2+ directly disrupts the actin assembly process, leading to altered actin organization such as branches and networks. Since the VSMC phenotype change and loss of contractility are fundamental to the development of atherosclerosis and related cardiovascular diseases, it is worth noting that these low doses of ZIF-8 nanoparticles administered intravenously could still be a safety concern in terms of cardiovascular risks. Moving forward, it is imperative to re-consider the "safe" nanoparticle dosages determined by biochemical cell assays alone, and take into account the impact of these nanoparticles on the biophysical characteristics of VSMCs, including changes in the actin cytoskeleton and cell morphology.
Keywords: Actin cytoskeleton; Cell morphology; Contractility; Vascular smooth muscle cells; Zeolitic imidazolate framework-8 nanoparticles.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures





References
-
- Wu M-X, Yang Y-W, Metal–Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy, Advanced Materials, 29 (2017) 1606134. - PubMed
-
- Wang Y, Yan J, Wen N, Xiong H, Cai S, He Q, Hu Y, Peng D, Liu Z, Liu Y, Metal-organic frameworks for stimuli-responsive drug delivery, Biomaterials, 230 (2020) 119619. - PubMed
-
- Zhang W, Lu J, Gao X, Li P, Zhang W, Ma Y, Wang H, Tang B, Enhanced Photodynamic Therapy by Reduced Levels of Intracellular Glutathione Obtained By Employing a Nano-MOF with CuII as the Active Center, Angewandte Chemie International Edition, 57 (2018) 4891–4896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

