Overexpressed WDR3 induces the activation of Hippo pathway by interacting with GATA4 in pancreatic cancer
- PMID: 33648545
- PMCID: PMC7923337
- DOI: 10.1186/s13046-021-01879-w
Overexpressed WDR3 induces the activation of Hippo pathway by interacting with GATA4 in pancreatic cancer
Abstract
Background: WD repeat domain 3 (WDR3) is involved in a variety of cellular processes including gene regulation, cell cycle progression, signal transduction and apoptosis. However, the biological role of WDR3 in pancreatic cancer and the associated mechanism remains unclear. We seek to explore the immune-independent functions and relevant mechanism for WDR3 in pancreatic cancer.
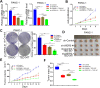
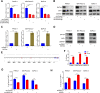
Methods: The GEPIA web tool was searched, and IHC assays were conducted to determine the mRNA and protein expression levels of WDR3 in pancreatic cancer patients. MTS, colony formation, and transwell assays were conducted to determine the biological role of WDR3 in human cancer. Western blot analysis, RT-qPCR, and immunohistochemistry were used to detect the expression of specific genes. An immunoprecipitation assay was used to explore protein-protein interactions.
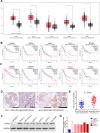
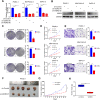
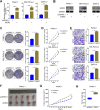
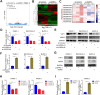
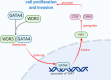
Results: Our study proved that overexpressed WDR3 was correlated with poor survival in pancreatic cancer and that WDR3 silencing significantly inhibited the proliferation, invasion, and tumor growth of pancreatic cancer. Furthermore, WDR3 activated the Hippo signaling pathway by inducing yes association protein 1 (YAP1) expression, and the combination of WDR3 silencing and administration of the YAP1 inhibitor TED-347 had a synergistic inhibitory effect on the progression of pancreatic cancer. Finally, the upregulation of YAP1 expression induced by WDR3 was dependent on an interaction with GATA binding protein 4 (GATA4), the transcription factor of YAP1, which interaction induced the nuclear translocation of GATA4 in pancreatic cancer cells.
Conclusions: We identified a novel mechanism by which WDR3 plays a critical role in promoting pancreatic cancer progression by activating the Hippo signaling pathway through the interaction with GATA4. Therefore, WDR3 is potentially a therapeutic target for pancreatic cancer treatment.
Keywords: GATA4; Hippo signaling pathway; Pancreatic Cancer; WDR3; YAP1.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. 10.3322/caac.21660. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

