tRNA sequences can assemble into a replicator
- PMID: 33648631
- PMCID: PMC7924937
- DOI: 10.7554/eLife.63431
tRNA sequences can assemble into a replicator
Abstract
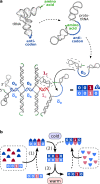
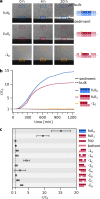
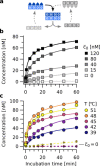
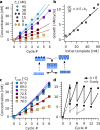
Can replication and translation emerge in a single mechanism via self-assembly? The key molecule, transfer RNA (tRNA), is one of the most ancient molecules and contains the genetic code. Our experiments show how a pool of oligonucleotides, adapted with minor mutations from tRNA, spontaneously formed molecular assemblies and replicated information autonomously using only reversible hybridization under thermal oscillations. The pool of cross-complementary hairpins self-selected by agglomeration and sedimentation. The metastable DNA hairpins bound to a template and then interconnected by hybridization. Thermal oscillations separated replicates from their templates and drove an exponential, cross-catalytic replication. The molecular assembly could encode and replicate binary sequences with a replication fidelity corresponding to 85-90 % per nucleotide. The replication by a self-assembly of tRNA-like sequences suggests that early forms of tRNA could have been involved in molecular replication. This would link the evolution of translation to a mechanism of molecular replication.
Keywords: Origin of tRNA; Self-assembly; computational biology; cross-catalytic replication; molecular biophysics; none; structural biology; systems biology.
Plain language summary
The genetic code stored within DNA contains the instructions for manufacturing all the proteins organisms need to develop, grow and survive. This requires molecular machines that ‘transcribe’ regions of the genetic code into RNA molecules which are then ‘translated’ into the string of amino acids that form the final protein. However, these molecular machines and other proteins are also needed to replicate and synthesize the sequences stored in DNA. This presents evolutionary biologists with a ‘chicken-and-egg’ situation: which came first, the DNA sequences needed to manufacture proteins or the proteins needed to transcribe and translate DNA? Understanding the order in which DNA replication and protein translation evolved is challenging as these processes are tightly intertwined in modern-day species. One theory, known as the ‘RNA world hypothesis’, suggests that all life on Earth began with a single RNA molecule that was able to make copies of itself, as DNA does today. To investigate this hypothesis, Kühnlein, Lanzmich and Braun studied a molecule called transfer RNA (or tRNA for short) which is responsible for translating RNA into proteins. tRNA is assumed to be one of the earliest evolved molecules in biology. Yet, why it was present in early life forms before it was needed for translation still remained somewhat of a mystery. To gain a better understanding of tRNA’s role early in evolution, Kühnlein, Lanzmich and Braun made small changes to its genetic code and then carried out tests on these tRNA-like sequences. The experiments showed these ‘early’ forms of tRNA can actually self-assemble into a molecule which is capable of replicating the information stored in its sequence. It suggests early forms of tRNA could have been involved in replication before modern tRNA developed its role in protein translation. With these experiments, Kühnlein, Lanzmich and Braun have identified a possible evolutionary link between DNA replication and protein translation, suggesting the two processes emerged through one shared pathway: tRNA. This deepens our understanding about the origins of early life, while taking biochemists one step closer to their distant goal of recreating self-replicating molecular machines in the laboratory.
© 2021, Kühnlein et al.
Conflict of interest statement
AK, SL, DB No competing interests declared
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

