Mapping immune variation and var gene switching in naive hosts infected with Plasmodium falciparum
- PMID: 33648633
- PMCID: PMC7924948
- DOI: 10.7554/eLife.62800
Mapping immune variation and var gene switching in naive hosts infected with Plasmodium falciparum
Abstract
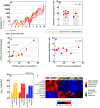
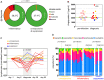
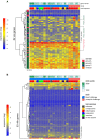
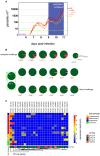
Falciparum malaria is clinically heterogeneous and the relative contribution of parasite and host in shaping disease severity remains unclear. We explored the interaction between inflammation and parasite variant surface antigen (VSA) expression, asking whether this relationship underpins the variation observed in controlled human malaria infection (CHMI). We uncovered marked heterogeneity in the host response to blood challenge; some volunteers remained quiescent, others triggered interferon-stimulated inflammation and some showed transcriptional evidence of myeloid cell suppression. Significantly, only inflammatory volunteers experienced hallmark symptoms of malaria. When we tracked temporal changes in parasite VSA expression to ask whether variants associated with severe disease rapidly expand in naive hosts, we found no transcriptional evidence to support this hypothesis. These data indicate that parasite variants that dominate severe malaria do not have an intrinsic growth or survival advantage; instead, they presumably rely upon infection-induced changes in their within-host environment for selection.
Keywords: P. falciparum; falciparum malaria; human; human immune variation; immunology; infectious disease; inflammation; metabolomics; microbiology; systems immunology; var gene switching.
© 2021, Milne et al.
Conflict of interest statement
KM, AI, AR, ML, AO, GS, DM, WN, CR, NE, SS, RP, AM, NV, MS, AH, MB, MB, SD, JR, PS No competing interests declared
Figures


References
-
- Abdi AI, Hodgson SH, Muthui MK, Kivisi CA, Kamuyu G, Kimani D, Hoffman SL, Juma E, Ogutu B, Draper SJ, Osier F, Bejon P, Marsh K, Bull PC. Plasmodium falciparum malaria parasite var gene expression is modified by host antibodies: longitudinal evidence from controlled infections of kenyan adults with varying natural exposure. BMC Infectious Diseases. 2017;17:585. doi: 10.1186/s12879-017-2686-0. - DOI - PMC - PubMed
-
- Bachmann A, Petter M, Krumkamp R, Esen M, Held J, Scholz JA, Li T, Sim BK, Hoffman SL, Kremsner PG, Mordmüller B, Duffy MF, Tannich E. Mosquito passage dramatically changes var gene expression in controlled human Plasmodium falciparum infections. PLOS Pathogens. 2016;12:e1005538. doi: 10.1371/journal.ppat.1005538. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

