Electrophysiological signatures of acute systemic lipopolysaccharide-induced inflammation: potential implications for delirium science
- PMID: 33648701
- PMCID: PMC8132883
- DOI: 10.1016/j.bja.2020.12.040
Electrophysiological signatures of acute systemic lipopolysaccharide-induced inflammation: potential implications for delirium science
Abstract
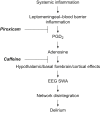
Background: Novel preventive therapies are needed for postoperative delirium, which especially affects older patients. A mouse model is presented that captures inflammation-associated cortical slow wave activity (SWA) observed in patients, allowing exploration of the mechanistic role of prostaglandin-adenosine signalling.
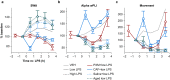
Methods: EEG and cortical cytokine measurements (interleukin 6, monocyte chemoattractant protein-1) were obtained from adult and aged mice. Behaviour, SWA, and functional connectivity were assayed before and after systemic administration of lipopolysaccharide (LPS)+piroxicam (cyclooxygenase inhibitor) or LPS+caffeine (adenosine receptor antagonist). To avoid the confounder of inflammation-driven changes in movement which alter SWA and connectivity, electrophysiological recordings were classified as occurring during quiescence or movement, and propensity score matching was used to match distributions of movement magnitude between baseline and post-LPS administration.
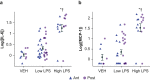
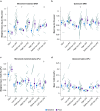
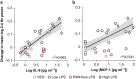
Results: LPS produces increases in cortical cytokines and behavioural quiescence. In movement-matched data, LPS produces increases in SWA (likelihood-ratio test: χ2(4)=21.51, P<0.001), but not connectivity (χ2(4)=6.39, P=0.17). Increases in SWA associate with interleukin 6 (P<0.001) and monocyte chemoattractant protein-1 (P=0.001) and are suppressed by piroxicam (P<0.001) and caffeine (P=0.046). Aged animals compared with adult animals show similar LPS-induced SWA during movement, but exaggerated cytokine response and increased SWA during quiescence.
Conclusions: Cytokine-SWA correlations during wakefulness are consistent with observations in patients with delirium. Absence of connectivity effects after accounting for movement changes suggests decreased connectivity in patients is a biomarker of hypoactivity. Exaggerated effects in quiescent aged animals are consistent with increased hypoactive delirium in older patients. Prostaglandin-adenosine signalling may link inflammation to neural changes and hence delirium.
Keywords: cytokines; delirium; electroencephalography; functional connectivity; slow wave activity.
Copyright © 2021 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Sanders R.D. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77:140–143. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

