No Time for Transcription-Rapid Auxin Responses in Plants
- PMID: 33648988
- PMCID: PMC8327833
- DOI: 10.1101/cshperspect.a039891
No Time for Transcription-Rapid Auxin Responses in Plants
Abstract
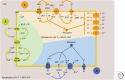
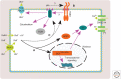
Auxin regulates the transcription of auxin-responsive genes by the TIR1/AFBs-Aux/IAA-ARF signaling pathway, and in this way facilitates plant growth and development. However, rapid, nontranscriptional responses to auxin that cannot be explained by this pathway have been reported. In this review, we focus on several examples of rapid auxin responses: (1) the triggering of changes in plasma membrane potential in various plant species and tissues, (2) inhibition of root growth, which also correlates with membrane potential changes, cytosolic Ca2+ spikes, and a rise of apoplastic pH, (3) the influence on endomembrane trafficking of PIN proteins and other membrane cargoes, and (4) activation of ROPs (Rho of plants) and their downstream effectors such as the cytoskeleton or vesicle trafficking. In most cases, the signaling pathway triggering the response is poorly understood. A role for the TIR1/AFBs in rapid root growth regulation is emerging, as well as the involvement of transmembrane kinases (TMKs) in the activation of ROPs. We discuss similarities and differences among these rapid responses and focus on their physiological significance, which remains an enigma in most cases.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Peŕet B, et al. 2012. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci 109: 4668–4673. 10.1073/pnas.1201498109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
