Rituximab or Cyclophosphamide in the Treatment of Membranous Nephropathy: The RI-CYCLO Randomized Trial
- PMID: 33649098
- PMCID: PMC8017548
- DOI: 10.1681/ASN.2020071091
Rituximab or Cyclophosphamide in the Treatment of Membranous Nephropathy: The RI-CYCLO Randomized Trial
Abstract
Background: A cyclic corticosteroid-cyclophosphamide regimen is the first-line therapy for membranous nephropathy. Compared with this regimen, rituximab therapy might have a more favorable safety profile, but a head-to-head comparison is lacking.
Methods: We randomly assigned 74 adults with membranous nephropathy and proteinuria >3.5 g/d to rituximab (1 g) on days 1 and 15, or a 6-month cyclic regimen with corticosteroids alternated with cyclophosphamide every other month. The primary outcome was complete remission of proteinuria at 12 months. Other outcomes included determination of complete or partial remission at 24 months and occurrence of adverse events.
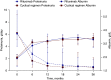
Results: At 12 months, six of 37 patients (16%) randomized to rituximab and 12 of 37 patients (32%) randomized to the cyclic regimen experienced complete remission (odds ratio [OR], 0.4; 95% CI, 0.13 to 1.23); 23 of 37 (62%) receiving rituximab and 27 of 37 (73%) receiving the cyclic regimen had complete or partial remission (OR, 0.61; 95% CI, 0.23 to 1.63). At 24 months, the probabilities of complete and of complete or partial remission with rituximab were 0.42 (95% CI, 0.26 to 0.62) and 0.83 (95% CI, 0.65 to 0.95), respectively, and 0.43 (95% CI, 0.28 to 0.61) and 0.82 (95% CI, 0.68 to 0.93), respectively, with the cyclic regimen. Serious adverse events occurred in 19% of patients receiving rituximab and in 14% receiving the cyclic regimen.
Conclusions: This pilot trial found no signal of more benefit or less harm associated with rituximab versus a cyclic corticosteroid-cyclophosphamide regimen in the treatment of membranous nephropathy. A head-to-head, pragmatic comparison of the cyclic regimen versus rituximab may require a global noninferiority trial.
Clinical trial registry name and registration number: Rituximab versus Steroids and Cyclophosphamide in the Treatment of Idiopathic Membranous Nephropathy (RI-CYCLO), NCT03018535.
Keywords: glomerulonephritis; membranous nephropathy; nephrotic syndrome.
Copyright © 2021 by the American Society of Nephrology.
Figures


References
-
- Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. .: Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97: 163–174, 2020 - PubMed
-
- Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, et al. .: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

