Population pharmacokinetics of dalbavancin and dosing consideration for optimal treatment of adult patients with staphylococcal osteoarticular infections
- PMID: 33649108
- PMCID: PMC8092885
- DOI: 10.1128/AAC.02260-20
Population pharmacokinetics of dalbavancin and dosing consideration for optimal treatment of adult patients with staphylococcal osteoarticular infections
Abstract
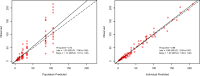
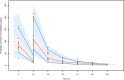
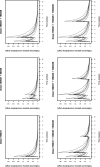
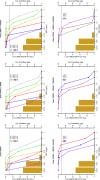
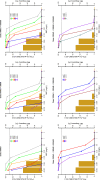
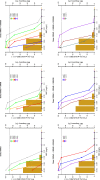
Background: Dalbavancin is gaining interest in the treatment of complex osteoarticular (OA) infections.Objective: To conduct a population pharmacokinetic analysis of dalbavancin in a prospective cohort of adult patients with Gram-positive OA infections and to identify optimal dosing regimens for long term-treatment.Methods: Non-linear mixed-effects modelling was performed with Monolix. Monte Carlo simulations were performed with six dalbavancin regimens (1500mg at day 1; 1000mg at day 1 plus 500mg at day 8; 1500mg at day1 and 8; 1500mg at day1 and 8 plus 500, 1000 or 1500mg at day 36) to assess the PTA of three pharmacodynamic target of fAUC24h/MIC against S. aureus (>27.1, 53.3 and 111.1). Cumulative fraction of response (CFR) was calculated against MIC distribution of both MRSA and MSSA as well. Desirable PTAs and CFRs were ≥90%.Results: Fifteen patients provided 120 plasma concentrations. Most (73.3%) had prosthetic joint infections. Clinical cure rate was 87%. A two-compartment model with linear elimination well described the data. No covariate was retained in the final model. Pharmacokinetic dalbavancin estimates were 0.106L/h for CL and 36.4L for Vss The tested dosing regimens granted desirable CFRs against S. aureus at the most effective PK/PD target for a period ranging 3-to-9 weeks. Conclusion: Giving a two 1500mg dosing regimen of dalbavancin one week apart may ensure efficacy against both MSSA and MRSA up to 5 weeks in patients with OA infections. Clinical assessment at that time may allow for considering whether or not an additional dose should be administered for prolonging effective treatment.
Copyright © 2021 American Society for Microbiology.
Figures
References
-
- Saeed K, Esposito S, Ascione T, Bassetti M, Bonnet E, Carnelutti A, Chan M, Lye DC, Cortes N, Dryden M, Fernando S, Gottlieb T, Gould I, Hijazi K, Madonia S, Pagliano P, Pottinger PS, Segreti J, Spera AM, International Society of Antimicrobial Chemotherapy (ISAC) Bone and Skin & Soft Tissue Infections Working Group . 2019. Hot topics on vertebral osteomyelitis from the International Society of Antimicrobial Chemotherapy. Int J Antimicrob Agents 54:125–133. 10.1016/j.ijantimicag.2019.06.013. - DOI - PubMed
-
- Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Hola V, Imbert C, Kirketerp-Moller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C, ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli . 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21(Suppl 1):S1–S25. 10.1016/j.cmi.2014.10.024. - DOI - PubMed
-
- Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, III, Petermann GW, Osmon DR, Infectious Diseases Society of America . 2015. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. 10.1093/cid/civ482. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

