Lytic bacteriophages facilitate antibiotic sensitization of Enterococcus faecium
- PMID: 33649110
- PMCID: PMC8092871
- DOI: 10.1128/AAC.00143-21
Lytic bacteriophages facilitate antibiotic sensitization of Enterococcus faecium
Erratum in
-
Correction for Canfield et al., "Lytic Bacteriophages Facilitate Antibiotic Sensitization of Enterococcus faecium".Antimicrob Agents Chemother. 2025 Aug 6;69(8):e0059025. doi: 10.1128/aac.00590-25. Epub 2025 Jul 8. Antimicrob Agents Chemother. 2025. PMID: 40626887 Free PMC article. No abstract available.
Abstract
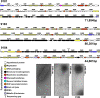
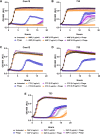
Enterococcus faecium, a commensal of the human intestine, has emerged as a hospital-adapted, multi-drug resistant (MDR) pathogen. Bacteriophages (phages), natural predators of bacteria, have regained attention as therapeutics to stem the rise of MDR bacteria. Despite their potential to curtail MDR E. faecium infections, the molecular events governing E. faecium-phage interactions remain largely unknown. Such interactions are important to delineate because phage selective pressure imposed on E. faecium will undoubtedly result in phage resistance phenotypes that could threaten the efficacy of phage therapy. In an effort to understand the emergence of phage resistance in E. faecium, three newly isolated lytic phages were used to demonstrate that E. faecium phage resistance is conferred through an array of cell wall-associated molecules, including secreted antigen A (SagA), enterococcal polysaccharide antigen (Epa), wall teichoic acids, capsule, and an arginine-aspartate-aspartate (RDD) protein of unknown function. We find that capsule and Epa are important for robust phage adsorption and that phage resistance mutations in sagA, epaR, and epaX enhance E. faecium susceptibility to ceftriaxone, an antibiotic normally ineffective due to its low affinity for enterococcal penicillin binding proteins. Consistent with these findings, we provide evidence that phages potently synergize with cell wall (ceftriaxone and ampicillin) and membrane-acting (daptomycin) antimicrobials to slow or completely inhibit the growth of E. faecium Our work demonstrates that the evolution of phage resistance comes with fitness defects resulting in drug sensitization and that lytic phages could serve as effective antimicrobials for the treatment of E. faecium infections.
Copyright © 2021 American Society for Microbiology.
Figures





References
-
- Kristich CJ, Rice LB, Arias CA. 2014. Enterococcal infection—treatment and antibiotic resistance, p 1–62. In Gilmore MS, Clewell DB, Ike Y, Shakar N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

