Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2
- PMID: 33649113
- PMCID: PMC8092915
- DOI: 10.1128/AAC.02428-20
Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2
Abstract
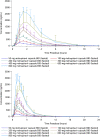
Molnupiravir, EIDD-2801/MK-4482, the prodrug of the active antiviral ribonucleoside analog ß-d-N4-hydroxycytidine (NHC; EIDD-1931), has activity against a number of RNA viruses including severe acute respiratory syndrome coronavirus 2, severe acute respiratory syndrome coronavirus, Middle East respiratory syndrome coronavirus, and seasonal and pandemic influenza viruses.Single and multiple doses of molnupiravir were evaluated in this first-in-human, phase 1, randomized, double-blind, placebo-controlled study in healthy volunteers, which included evaluation of the effect of food on pharmacokinetics.EIDD-1931 appeared rapidly in plasma, with a median time of maximum observed concentration of 1.00 to 1.75 hours, and declined with a geometric half-life of approximately 1 hour, with a slower elimination phase apparent following multiple doses or higher single doses (7.1 hours at the highest dose tested). Mean maximum observed concentration and area under the concentration versus time curve increased in a dose-proportional manner, and there was no accumulation following multiple doses. When administered in a fed state, there was a decrease in the rate of absorption, but no decrease in overall exposure.Molnupiravir was well tolerated. Fewer than half of subjects reported an adverse event, the incidence of adverse events was higher following administration of placebo, and 93.3% of adverse events were mild. One discontinued early due to rash. There were no serious adverse events and there were no clinically significant findings in clinical laboratory, vital signs, or electrocardiography. Plasma exposures exceeded expected efficacious doses based on scaling from animal models; therefore, dose escalations were discontinued before a maximum tolerated dose was reached.
Copyright © 2021 Painter et al.
Figures
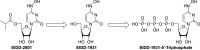


References
-
- World Health Organization. 2020. Novel coronavirus (2019-nCoV) situation report 1. World Health Organization, Geneva, Switzerland.
-
- Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, Leist SR, Schäfer A, Dinnon KH, Stevens LJ, Chappell JD, Lu X, Hughes TM, George AS, Hill CS, Montgomery SA, Brown AJ, Bluemling GR, Natchus MG, Saindane M, Kolykhalov AA, Painter G, Harcourt J, Tamin A, Thornburg NJ, Swanstrom R, Denison MR, Baric RS. 2020. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus. Sci Transl Med 12:eabb5883. doi:10.1126/scitranslmed.abb5883. - DOI - PMC - PubMed
-
- Toots M, Yoon J-J, Cox RM, Hart M, Sticher ZM, Makhsous N, Plesker R, Barrena AH, Reddy PG, Mitchell DG, Shean RC, Bluemling GR, Kolykhalov AA, Greninger AL, Natchus MG, Painter GR, Plemper RK. 2019. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med 11:eaax5866. doi:10.1126/scitranslmed.aax5866. - DOI - PMC - PubMed
-
- Painter GR, Bowen RA, Bluemling GR, DeBergh J, Edpuganti V, Gruddanti PR, Guthrie DB, Hager M, Kuiper DL, Lockwood MA, Mitchell DG, Natchus MG, Sticher ZM, Kolykhalov AA. 2019. The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal Venezuelan equine encephalitis virus infection. Antiviral Res 171:104597. doi:10.1016/j.antiviral.2019.104597. - DOI - PubMed
-
- Agostini ML, Pruijssers AJ, Chappell JD, Gribble J, Lu X, Andres EL, Bluemling GR, Lockwood MA, Sheahan TP, Sims AC, Natchus MG, Saindane M, Kolykhalov AA, Painter GR, Baric RS, Denison MR. 2019. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol 93:e01348-19. doi:10.1128/JVI.01348-19. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

