Evolution of Ribosomal Protein S14 Demonstrated by the Reconstruction of Chimeric Ribosomes in Bacillus subtilis
- PMID: 33649148
- PMCID: PMC8088600
- DOI: 10.1128/JB.00599-20
Evolution of Ribosomal Protein S14 Demonstrated by the Reconstruction of Chimeric Ribosomes in Bacillus subtilis
Abstract
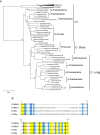
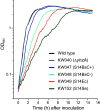
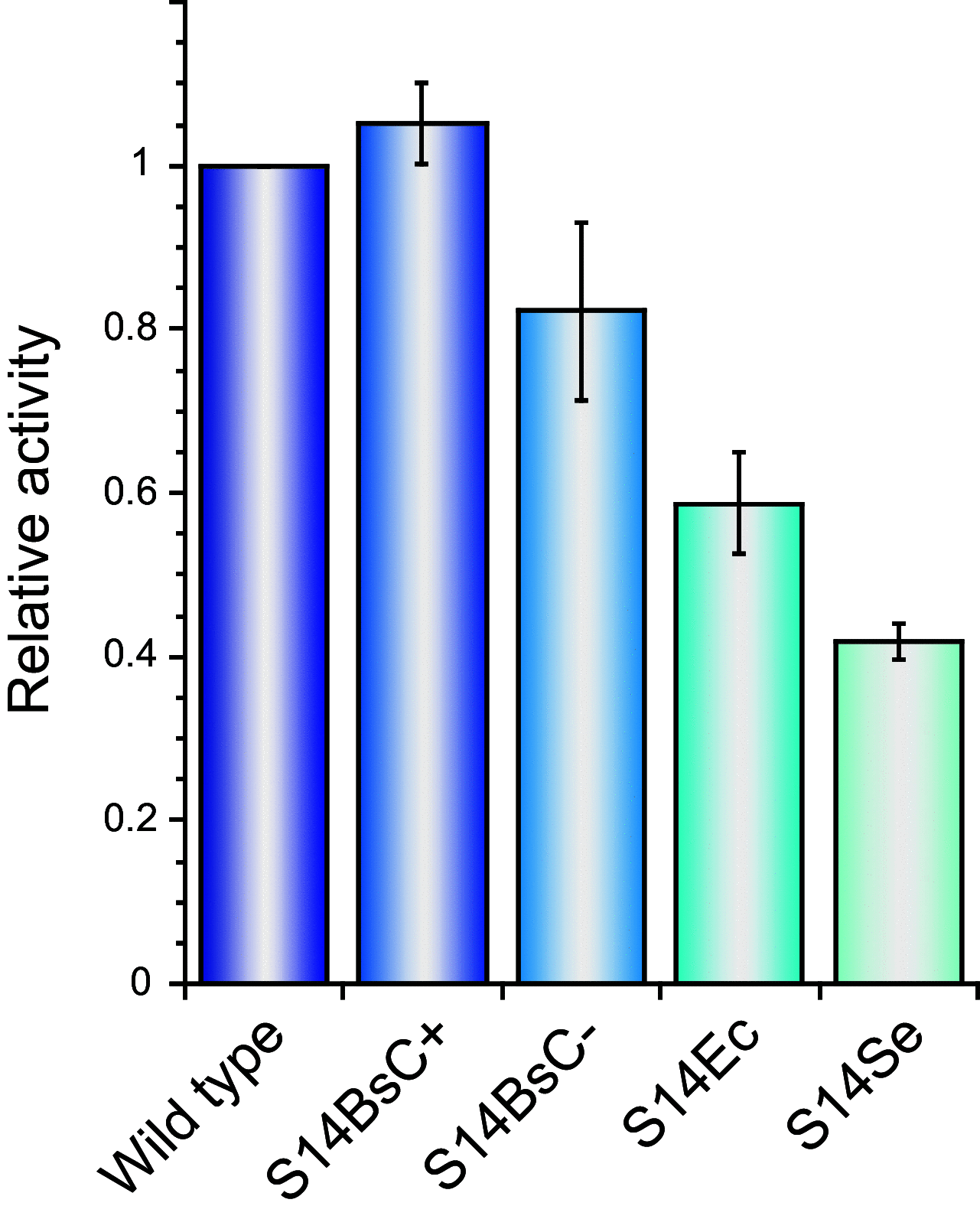
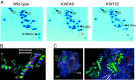
Ribosomal protein S14 can be classified into three types. The first, the C+ type has a Zn2+ binding motif and is ancestral. The second and third are the C- short and C- long types, neither of which contain a Zn2+ binding motif and which are ca. 90 residues and 100 residues in length, respectively. In the present study, the C+ type S14 from Bacillus subtilis ribosomes (S14BsC+) were completely replaced by the heterologous C- long type of S14 from Escherichia coli (S14Ec) or Synechococcus elongatus (S14Se). Surprisingly, S14Ec and S14Se were incorporated fully into 70S ribosomes in B. subtilis However, the growth rates as well as the sporulation efficiency of the mutants harboring heterologous S14 were significantly decreased. In these mutants, the polysome fraction was decreased and the 30S and 50S subunits accumulated unusually, indicating that cellular translational activity of these mutants was decreased. In vitro analysis showed a reduction in the translational activity of the 70S ribosome fraction purified from these mutants. The abundance of ribosomal proteins S2 and S3 in the 30S fraction in these mutants was reduced while that of S14 was not significantly decreased. It seems likely that binding of heterologous S14 changes the structure of the 30S subunit, which causes a decrease in the assembly efficiency of S2 and S3, which are located near the binding site of S14. Moreover, we found that S3 from S. elongatus cannot function in B. subtilis unless S14Se is present.IMPORTANCE S14, an essential ribosomal protein, may have evolved to adapt bacteria to zinc-limited environments by replacement of a zinc-binding motif with a zinc-independent sequence. It was expected that the bacterial ribosome would be tolerant to replacement of S14 because of the previous prediction that the spread of C- type S14 involved horizontal gene transfer. In this study, we completely replaced the C+ type of S14 in B. subtilis ribosome with the heterologous C- long type of S14 and characterized the resulting chimeric ribosomes. Our results suggest that the B. subtilis ribosome is permissive for the replacement of S14, but coevolution of S3 might be required to utilize the C- long type of S14 more effectively.
Keywords: Bacillus subtilis; ribosomal protein S14; ribosome; zinc.
Copyright © 2021 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

