Mitochondria
- PMID: 33649187
- PMCID: PMC7919390
- DOI: 10.1101/cshperspect.a040543
Mitochondria
Figures



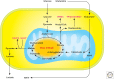







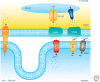

References
-
- Chance B, Williams GR. 1955. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 217: 409–427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
