Structural insights into α-synuclein monomer-fibril interactions
- PMID: 33649211
- PMCID: PMC7958257
- DOI: 10.1073/pnas.2012171118
Structural insights into α-synuclein monomer-fibril interactions
Abstract
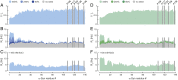
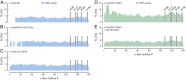
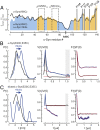
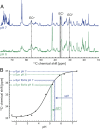
Protein aggregation into amyloid fibrils is associated with multiple neurodegenerative diseases, including Parkinson's disease. Kinetic data and biophysical characterization have shown that the secondary nucleation pathway highly accelerates aggregation via the absorption of monomeric protein on the surface of amyloid fibrils. Here, we used NMR and electron paramagnetic resonance spectroscopy to investigate the interaction of monomeric α-synuclein (α-Syn) with its fibrillar form. We demonstrate that α-Syn monomers interact transiently via their positively charged N terminus with the negatively charged flexible C-terminal ends of the fibrils. These intermolecular interactions reduce intramolecular contacts in monomeric α-Syn, yielding further unfolding of the partially collapsed intrinsically disordered states of α-Syn along with a possible increase in the local concentration of soluble α-Syn and alignment of individual monomers on the fibril surface. Our data indicate that intramolecular unfolding critically contributes to the aggregation kinetics of α-Syn during secondary nucleation.
Keywords: Parkinson’s disease; protein aggregation; secondary nucleation; α-synuclein.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Spillantini M. G., et al. ., Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997). - PubMed
-
- Spillantini M. G., Goedert M., The α-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann. N. Y. Acad. Sci. 920, 16–27 (2000). - PubMed
-
- Krüger R., et al. ., Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 18, 106–108 (1998). - PubMed
-
- Zarranz J. J., et al. ., The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 (2004). - PubMed
-
- Appel-Cresswell S., et al. ., Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov. Disord. 28, 811–813 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

