HIV-1 cores retain their integrity until minutes before uncoating in the nucleus
- PMID: 33649225
- PMCID: PMC7958386
- DOI: 10.1073/pnas.2019467118
HIV-1 cores retain their integrity until minutes before uncoating in the nucleus
Abstract
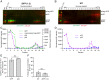
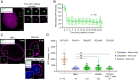
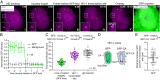
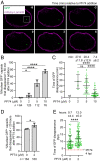
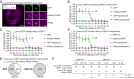
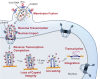
We recently reported that HIV-1 cores that retained >94% of their capsid (CA) protein entered the nucleus and disassembled (uncoated) near their integration site <1.5 h before integration. However, whether the nuclear capsids lost their integrity by rupturing or a small loss of CA before capsid disassembly was unclear. Here, we utilized a previously reported vector in which green fluorescent protein is inserted in HIV-1 Gag (iGFP); proteolytic processing efficiently releases GFP, some of which remains trapped inside capsids and serves as a fluid phase content marker that is released when the capsids lose their integrity. We found that nuclear capsids retained their integrity until shortly before integration and lost their GFP content marker ∼1 to 3 min before loss of capsid-associated mRuby-tagged cleavage and polyadenylation specificity factor 6 (mRuby-CPSF6). In contrast, loss of GFP fused to CA and mRuby-CPSF6 occurred simultaneously, indicating that viral cores retain their integrity until just minutes before uncoating. Our results indicate that HIV-1 evolved to retain its capsid integrity and maintain a separation between macromolecules in the viral core and the nuclear environment until uncoating occurs just before integration. These observations imply that intact HIV-1 capsids are imported through nuclear pores; that reverse transcription occurs in an intact capsid; and that interactions between the preintegration complex and LEDGF/p75, and possibly other host factors that facilitate integration, must occur during the short time period between loss of capsid integrity and integration.
Keywords: HIV-1; capsid; core integrity; nuclear import; uncoating.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Mattei S., Glass B., Hagen W. J., Kräusslich H. G., Briggs J. A., The structure and flexibility of conical HIV-1 capsids determined within intact virions. Science 354, 1434–1437 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

