Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3
- PMID: 33649229
- PMCID: PMC7958351
- DOI: 10.1073/pnas.2020401118
Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3
Abstract
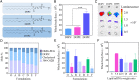
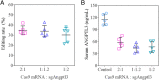
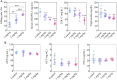
Loss-of-function mutations in Angiopoietin-like 3 (Angptl3) are associated with lowered blood lipid levels, making Angptl3 an attractive therapeutic target for the treatment of human lipoprotein metabolism disorders. In this study, we developed a lipid nanoparticle delivery platform carrying Cas9 messenger RNA (mRNA) and guide RNA for CRISPR-Cas9-based genome editing of Angptl3 in vivo. This system mediated specific and efficient Angptl3 gene knockdown in the liver of wild-type C57BL/6 mice, resulting in profound reductions in serum ANGPTL3 protein, low density lipoprotein cholesterol, and triglyceride levels. Our delivery platform is significantly more efficient than the FDA-approved MC-3 LNP, the current gold standard. No evidence of off-target mutagenesis was detected at any of the nine top-predicted sites, and no evidence of toxicity was detected in the liver. Importantly, the therapeutic effect of genome editing was stable for at least 100 d after a single dose administration. This study highlights the potential of LNP-mediated delivery as a specific, effective, and safe platform for Cas9-based therapeutics.
Keywords: Angptl3; CRISPR-Cas9 mRNA delivery; genome editing; lipid nanoparticles.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Koishi R., et al., Angptl3 regulates lipid metabolism in mice. Nat. Genet. 30, 151–157 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

