Cerebral functional networks during sleep in young and older individuals
- PMID: 33649377
- PMCID: PMC7921592
- DOI: 10.1038/s41598-021-84417-0
Cerebral functional networks during sleep in young and older individuals
Abstract
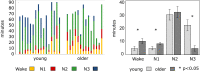
Even though sleep modification is a hallmark of the aging process, age-related changes in functional connectivity using functional Magnetic Resonance Imaging (fMRI) during sleep, remain unknown. Here, we combined electroencephalography and fMRI to examine functional connectivity differences between wakefulness and light sleep stages (N1 and N2 stages) in 16 young (23.1 ± 3.3y; 7 women), and 14 older individuals (59.6 ± 5.7y; 8 women). Results revealed extended, distributed (inter-between) and local (intra-within) decreases in network connectivity during sleep both in young and older individuals. However, compared to the young participants, older individuals showed lower decreases in connectivity or even increases in connectivity between thalamus/basal ganglia and several cerebral regions as well as between frontal regions of various networks. These findings reflect a reduced ability of the older brain to disconnect during sleep that may impede optimal disengagement for loss of responsiveness, enhanced lighter and fragmented sleep, and contribute to age effects on sleep-dependent brain plasticity.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

